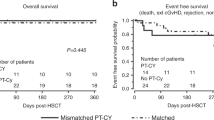
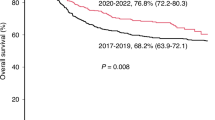
Abstract
Primary immunodeficiencies constitute a group of highly complex congenital disorders most of which are characterized by poor prognosis with high mortality and morbidity. Hematopoietic SCT became an established therapy for such disorders. The first clear-cut report of successful allogenic SCT in 1968 dealt with the treatment of a patient with primary immunodeficiency, that is, SCID and Wiskott–Aldrich syndrome. Starting with this pioneering experience in 1968, hundreds of SCID patients and hundreds of patients affected by other life-threatening forms of primary immunodeficiency throughout the world have benefited from SCT. Presently, hematopoietic SCT from an HLA-matched sibling donor confers at least 80% chance of cure for children affected by primary immunodeficiency and about a 70% chance of cure when a fully HLA-matched related donor is available. This high success rate is the consequence of better management of nutrition and the infection problem affecting these patients at the time of disease. Conversely, when a related HLA-mismatched donor is used, the survival rate is significantly lower than that of patients receiving SCT from either an HLA-matched sibling or a fully matched HLA-unrelated donor. Optimal results and outcome of SCT are highly dependent on early and correct diagnosis of these disorders. SCT should be applied early in the course of the disease to prevent irreversible complications from the primary disease and/or infection. We present the data on outcome for primary immunodeficiency transplantation at King Faisal Specialist Hospital from 1993 to 2006.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bach FH, Albertini RJ, Joo P, Anderson JL, Bortin MD . Bone marrow transplantation in a patient with the Wiskott–Aldrich syndrome. Lancet 1968; 2: 1364–1366.
Amos DB, Bach FH . Phenotypic expressions of the major histocompatibility locus in a man (HL-A): leukocyte antigens and mixed leukocyte culture reactivity. J Exp Med 1968; 128: 623–637.
Suliaman F, Al-Ghonaium A, Harfi H . High incidence of severe combined immune deficiency in the Eastern province of Saudi Arabia. Pediatr Asthma Aller Immunol 2006; 19: 14–17.
Cavazzana-Calvo M, Lagresle C, Hacein-Bey-Abina S, Fischer A . Gene therapy for severe combined immunodeficiency. Ann Rev Med 2005; 56: 585–602.
Gaspar B, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J et al. Successful gene therapy of SCID-X1 using a pseudotyped gammaretroviral vector. Lancet 2004; 364: 2181–2187.
Antoine C, Muller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J et al. Long-term survival and hematopoietic stem-cell transplantation for immunodeficiencies. A survey of the European experience. Lancet 2003; 361: 553–560.
Cavazzana-Calvo M, Fischer A . Gene therapy for severe combined immunodeficiency: are we there yet? J Clin Invest 2007; 117: 1456–1465.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Ghonaium, A. Stem cell transplantation for primary immunodeficiencies: King Faisal Specialist Hospital experience from 1993 to 2006. Bone Marrow Transplant 42 (Suppl 1), S53–S56 (2008). https://doi.org/10.1038/bmt.2008.115
Published:
Issue date:
DOI: https://doi.org/10.1038/bmt.2008.115
Keywords
This article is cited by
-
Alternative donor SCT for the treatment of MHC Class II deficiency
Bone Marrow Transplantation (2013)