Abstract
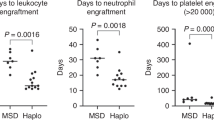
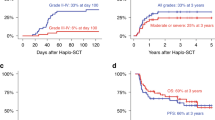
Haploidentical SCT (HaploSCT) has been most commonly performed using a myeloablative, TBI-based preparative regimen; however, the toxicity with this approach remains very high. We studied the feasibility of a reduced-intensity conditioning regimen in a phase II clinical trial using fludarabine, melphalan and thiotepa and antithymocyte globulin (ATG) for patients with advanced hematological malignancies undergoing T-cell depleted HaploSCT. Twenty-eight patients were entered in the study. Engraftment with donor-derived hematopoiesis was achieved in 78% of patients after a median of 13 days. Six patients experienced primary graft failure, three out of four tested patients had donor-specific anti-HLA antibodies (DSA) (P=0.001). Toxicity included mostly infections. A total of 21 out of 22 patients with AML/myelodysplastic syndrome (MDS) achieved remission after transplant (16 with relapsed/refractory AML). Five out of the 12 patients (42%) with AML/MDS with <15% BM blasts survived long term as compared with none with more advanced disease (P=0.03). HaploSCT with this fludarabine, melphalan and thiotepa and ATG RIC is an effective, well-tolerated conditioning regimen for patients with AML/MDS with low disease burden at the time of transplant and allowed a high rate of engraftment in patients without DSA. Patients with overt relapse fared poorly and require novel treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Thomas ED, Buckner CD, Banaji M, Clift RA, Fefer A, Flournoy N et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood 1977; 49: 1511–1533.
Copelan EA . Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–1826.
Beatty PG, Mori M, Milford E . Impact of racial genetic polymorphism on the probability of finding an HLA-matched donor. Transplantation 1995; 60: 778–783.
Schipper RF, D’Amaro J, Bakker JT, Bakker J, van Rood JJ, Oudshoorn M . HLA gene haplotype frequencies in bone marrow donors worldwide registries. Hum Immunol 1997; 52: 54–71.
Powles RL, Morgenstern GR, Kay HE, McElwain TJ, Clink HM, Dady PJ et al. Mismatched family donors for bone marrow transplantation as treatment for acute leukemia. Lancet 1983; 8325: 612–615.
Marmont AM, Horowitz MM, Gale RP, Sobocinski K, Ash RC, van Bekkum DW, Champlin RE et al. T-cell depletion of HLA-identical transplants in leukemia. Blood 1991; 78: 2120–2130.
Reisner Y, Kapoor N, Kirkpatrick D, Pollack MS, Cunningham-Rundles S, Dupont B et al. Transplantation for severe combined immunodeficiency with HLA-A, B, D, DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood 1983; 61: 341–348.
Aversa F, Tabilio A, Terenzi A, Velardi A, Falzetti F, Giannoni C et al. Successful engraftment of T-cell-depleted haploidentical ‘three-loci’ incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood 1994; 84: 3948–3955.
Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA Haplotype. N Engl J Med 1998; 339: 1186–1193.
Aversa F, Terenzi A, Felicini R, Carotti A, Falcinelli F, Tabilio A et al. Haploidentical stem cell transplantation for acute leukemia. Int J Hematol 2002; 76: 165–168.
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol 2005; 23: 3447–3454.
Champlin R, Hesdorffer C, Lowenberg B, Martelli MF, Martelsmann RH, Reisner Y et al. Haploidentical ‘megadose’ stem cell transplantation in acute leukemia: recommendations for a protocol agreed upon at the Perugia and Chicago meetings. Leukemia 2002; 16: 427–428.
O’Reilly RJ . T-cell depletion and allogeneic bone marrow transplantation. Semin Hematol 1992; 29: 20–26.
Handgretinger R, Klingebiel T, Lang P, Schumm M, Neu S, Geiselhart A et al. Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant 2001; 27: 777–783.
Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH et al. Results of allogeneic bone marrow transplant for leukemia using donors other then HLA-identical sibling. J Clin Oncol 1997; 15: 1767–1777.
Kato S, Yabe H, Yasui M, Kawa K, Yoshida T, Watanabe A et al. Allogeneic hematopoietic transplantation of CD34+ selected cells from an HLA haploidentical related donor. Along-term follow-up of 135 patients and a comparison of stem cell source between the bone marrow and the peripheral blood. Bone Marrow Transplant 2000; 26: 1281–1290.
Dobyski WR, Klein J, Flomenberg N, Pietryga D, Vesole DH, Margolis DA et al. Superior survival associated with transplantation of matched unrelated versus on-antigen mismatched unrelated or highly human-leukocyte antigen-disparate haploidentical family donor marrow grafts for the treatment of hematologic malignancies: establishing a treatment algorithm for recipients of alternative donor grafts. Blood 2002; 99: 806–814.
Kanda Y, Chiba S, Hirai H, Sakamaki H, Iseki T, Kodera Y et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991–2000). Blood 2003; 102: 1541–1547.
Mehta J, Singhal S, Gee AP, Chiang KY, Godder K, Rhee Fv F et al. Bone marrow transplantation from partially HLA-mismatched family donors for acute leukemia: single-center experience of 201 patients. Bone Marrow Transplant 2004; 33: 389–396.
Koh LP, Rizzieri DA, Chao NJ . Allogeneic hematopoietic stem cell transplant using mismatched/haploidentical donors. Biol Blood Marrow Transplant 2007; 13: 1249–1267.
Giralt S, Estey E, Albitar M, van Besien K, Rondon G, Anderlini P et al. Engraftment of allogneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood 1997; 89: 4531–4536.
Spitzer TR, McAfee SL, Dey BR, Colby C, Hope J, Grossberg H et al. Nonmyeloablative haploidentical stem-cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation 2003; 75: 1448–1751.
Rizzieri DA, Koh LP, Long GD, Gasparetto C, Sullivan KM, Horwitz M et al. Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. J Clin Oncol 2007; 25: 690–697.
Miltenyi S . Isolation of CD34+ Hematopoietic Progenitor Cells By High-Gradient Magnetic Cell Sorting (MACS). In: Wunder E (ed). Hematopoietic Stem Cells: The Mullhouse Manual. AlphaMed Press: Dayton, OH, 1994, 201–213.
Thiede C, Florek M, Bornhauser M, Ritter M, Mohr B, Brendel C et al. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant 1999; 23: 1055–1060.
Przepiorka D, Martin P, Klingmann HG, Beatty P, Hows J, Thomas ED . 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol 1991; 28: 250–259.
Common Toxicity Criteria Version 3 0 Available at http://ctep.info.nih.gov/reporting/ctc.html.
Kaplan EL, Meier P . Nonparametric estimation for incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Prentice RL, Kalbfleisch JD, Peterson Jr AV, Flournoy N, Farewell VT, Breslow NE . The analysis of failure times in the presence of competing risks. Biometrics 1978; 34: 541–554.
Cox DR . Regression models and life tables [with discussion]. J R Stat Soc B 1972; 34: 187–202.
Ruggeri L, Capanni M, Tosti A, Perruccio K, Shlomchik WD, Tosti A et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295: 2097–2100.
Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT et al. Donors with group B KiR haplotypes improve relapse-free survival after unrelated hematopoietic stem cell transplantation for acute myelogenous leukemia. Blood 2009; 113: 726–731.
van Rood JJ, Loberiza Jr FR, Zhang MJ, Oudshoorn M, Claas F, Cairo MS et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or and HLA-haploidentical sibling. Blood 2002; 99: 1572–1577.
Lacerda JF, Martins C, Carmo JA, Lourenço F, Juncal C, Rodrigues A et al. Haploidentical stem cell transplantation with purified CD34+ cells after a chemotherapy-alone conditioning regimen. Biol Blood Marrow Transplant 2003; 9: 633–642.
Bethge WA, Haegele M, Faul C, Lang P, Schumm M, Bornhauser M et al. Haploidentical allogeneic stem cell transplantation in adults with reduced-intensity conditioning and CD3/CD19 depletion: fast engraftment and low toxicity. Exp Hematol 2006; 34: 1746–1752.
Handgretinger R, Chen X, Pfeiffer M, Mueller I, Feuchtinger T, Hale GA et al. Feasibility and outcome of reduced-intensity conditioning in haploidentical transplantation. Ann N Y Acad Sci 2007; 1106: 279–289.
Ciceri F, Lobopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood 2008; 112: 3574–3581.
Acknowledgements
We thank all our pharmacists, transplant coordinators and nursing staff for their dedication to patient care; research nurses, technicians and laboratory personnel who contributed to the collection, recording and reporting of the data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ciurea, S., Saliba, R., Rondon, G. et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant 45, 429–436 (2010). https://doi.org/10.1038/bmt.2009.189
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/bmt.2009.189
Keywords
This article is cited by
-
Comparison of fludarabine/melphalan (FluMel) with fludarabine/melphalan/BCNU or thiotepa (FBM/FTM) in patients with AML in first complete remission undergoing allogeneic hematopoietic stem cell transplantation – a registry study on behalf of the EBMT Acute Leukemia Working Party
Bone Marrow Transplantation (2024)
-
Comparison of reduced-toxicity conditioning protocols using fludarabine, melphalan combined with thiotepa or carmustine in allogeneic hematopoietic cell transplantation
Bone Marrow Transplantation (2021)
-
The European Society for Blood and Marrow Transplantation (EBMT) consensus recommendations for donor selection in haploidentical hematopoietic cell transplantation
Bone Marrow Transplantation (2020)
-
Graft versus host disease in unmanipulated haploidentical marrow transplantation with a modified post-transplant cyclophosphamide (PT-CY) regimen: an update on 425 patients
Bone Marrow Transplantation (2019)
-
Pilot study using post-transplant cyclophosphamide (PTCy), tacrolimus and mycophenolate GVHD prophylaxis for older patients receiving 10/10 HLA-matched unrelated donor hematopoietic stem cell transplantation
Bone Marrow Transplantation (2019)