Abstract
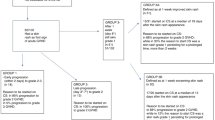
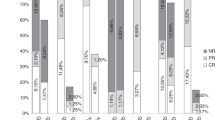
One of the obstacles to chronic GVHD research is the lack of standardized response criteria. The National Institute of Health (NIH) has recommended response criteria at a Consensus Conference. These need to be validated. We recently completed and reported a trial of pentostatin in treating steroid-refractory chronic GVHD. During the trial, we prospectively collected percent body-surface-area (BSA) involvement of rash, superficial sclerosis and deep sclerosis. Here, we compare cutaneous responses using the NIH scale and the Hopkins scale. The two scales produced similar overall response rates but different domain response rates. There was 80% agreement in overall response at the final treatment evaluation, but only a 64% agreement for fasciitis/non-moveable sclerosis. There was more disparity in the measurement of sclerosis than in that of erythema, which highlights the difficulty of quantifying sclerosis. For sclerosis, the Hopkins scale, which used skin softening, was more predictive of early response as compared with the NIH scale, which focused on percent BSA. Early assessment of skin softening may be important if trying to detect the activity of a particular agent in chronic GVHD. Further validation of the NIH scale is ongoing, which should produce a clinically useful and predictive scale.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kim JG, Sohn SK, Kim DH, Lee NY, Suh JS, Lee KS et al. Different efficacy of mycophenolate mofetil as salvage treatment for acute and chronic GVHD after allogeneic stem cell transplant. Eur J Haematol 2004; 73: 56–61.
Couriel DR, Hosing C, Saliba R, Shpall EJ, Anderlini P, Rhodes B et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood 2006; 107: 3074–3080.
Johnston LJ, Brown J, Shizuru JA, Stockerl-Goldstein KE, Stuart MJ, Blume KG et al. Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2005; 11: 47–55.
Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood 2006; 108: 756–762.
Jacobsohn DA, Chen AR, Zahurak M, Piantadosi S, Anders V, Bolanos-Meade J et al. Phase II study of pentostatin in patients with corticosteroid-refractory chronic graft-versus-host disease. J Clin Oncol 2007; 25: 4255–4261.
Flowers ME, Apperley JF, van Besien K, Elmaagacli A, Grigg A, Reddy V et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood 2008; 112: 2667–2674.
Jagasia M, Giglia J, Chinratanalab W, Dixon S, Chen H, Frangoul H et al. Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health Consensus criteria. Biol Blood Marrow Transplant 2007; 13: 1207–1215.
Arora M, Nagaraj S, Witte J, Defor TE, Macmillan M, Burns LJ et al. New classification of chronic GVHD: added clarity from the consensus diagnoses. Bone Marrow Transplant 2009; 43: 149–153.
Cho BS, Min CK, Eom KS, Kim YJ, Kim HJ, Lee S et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia 2009; 23: 78–84.
Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 2006; 12: 252–266.
Greinix HT, Pohlreich D, Maalouf J, Soukup P, Supper V, Kalhs P et al. A single-center pilot validation study of a new chronic GVHD skin scoring system. Biol Blood Marrow Transplant 2007; 13: 715–723.
Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol 1995; 22: 1281–1285.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacobsohn, D., Rademaker, A., Kaup, M. et al. Skin response using NIH consensus criteria vs Hopkins scale in a phase II study for steroid-refractory chronic GVHD. Bone Marrow Transplant 44, 813–819 (2009). https://doi.org/10.1038/bmt.2009.84
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/bmt.2009.84
Keywords
This article is cited by
-
Graft Versus Host Disease Clinical Trials: Is it Time for Patients Centered Outcomes to Be the Primary Objective?
Current Hematologic Malignancy Reports (2019)