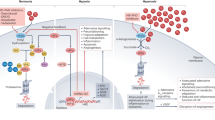
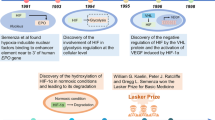
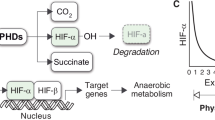
Abstract
The increase in body size of humans and other vertebrates requires a physiological infrastructure to provide adequate delivery of oxygen to tissues and cells to maintain oxygen homeostasis. The heart, lungs and the vasculature are all part of a highly regulated system that ensures the distribution of the precise amount of oxygen needed throughout the mammalian organism. Given its fundamental impact on physiology and pathology, it is no surprise that the response of cells to a lack of oxygen, termed hypoxia, has been the focus of many research groups worldwide for many decades now. The transcriptional complex hypoxia-inducible factor has emerged as a key regulator of the molecular hypoxic response, mediating a wide range of physiological and cellular mechanisms necessary to adapt to reduced oxygen.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- EPO:
-
erythropoietin
- HIF:
-
hypoxia-inducible factor
- ODDD:
-
oxygen-dependent degradation domain
- pVHL:
-
von Hippel–Lindau tumor suppressor protein
References
Semenza GL, Wang GL . A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 1992; 12: 5447–5454.
Maxwell PH, Pugh CW, Ratcliffe PJ . Activation of the HIF pathway in cancer. Curr Opin Genet Dev 2001; 11: 293–299.
Wang GL, Jiang BH, Rue EA, Semenza GL . Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995; 92: 5510–5514.
Gu YZ, Hogenesch JB, Bradfield CA . The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol 2000; 40: 519–561.
Jewell UR, Kvietikova I, Scheid A, Bauer C, Wenger RH, Gassmann M . Induction of HIF-1alpha in response to hypoxia is instantaneous. FASEB J 2001; 15: 1312–1314.
Jiang BH, Semenza GL, Bauer C, Marti HH . Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 1996; 271 (4 Pt 1): C1172–C1180.
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001; 292: 464–468.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ et al. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001; 292: 468–472.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999; 399: 271–275.
Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel–Lindau protein. Nat Cell Biol 2000; 2: 423–427.
Bruick RK, McKnight SL . A conserved family of prolyl-4-hydroxylases that modify HIF. Science 2001; 294: 1337–1340.
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR et al. C.elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001; 107: 43–54.
Huang LE, Gu J, Schau M, Bunn HF . Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin–proteasome pathway. Proc Natl Acad Sci USA 1998; 95: 7987–7992.
Pugh CW, O'Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ . Activation of hypoxia-inducible factor-1; definition of regulatory domains within the alpha subunit. J Biol Chem 1997; 272: 11205–11214.
Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ . Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J 2001; 20: 5197–5206.
Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL . Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem 1997; 272: 19253–19260.
Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML . Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 2002; 295: 858–861.
Hewitson KS, McNeill LA, Riordan MV, Tian YM, Bullock AN, Welford RW et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem 2002; 277: 26351–26355.
Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK . FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 2002; 16: 1466–1471.
Mahon PC, Hirota K, Semenza GL . FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 2001; 15: 2675–2686.
Iliopoulos O, Levy AP, Jiang C, Kaelin Jr WG, Goldberg MA . Negative regulation of hypoxia-inducible genes by the von Hippel–Lindau protein. Proc Natl Acad Sci USA 1996; 93: 10595–10599.
Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J 2003; 17: 271–273.
Hu CJ, Sataur A, Wang L, Chen H, Simon MC . The N-terminal transactivation domain confers target gene specificity of hypoxia inducible factors HIF-1 alpha and HIF-2 alpha. Mol Biol Cell 2007; 18: 4528–4542.
Lau KW, Tian YM, Raval RR, Ratcliffe PJ, Pugh CW . Target gene selectivity of hypoxia-inducible factor-alpha in renal cancer cells is conveyed by post-DNA-binding mechanisms. Br J Cancer 2007; 96: 1284–1292.
Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 2001; 414: 550–554.
Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ . Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc Natl Acad Sci USA 1994; 91: 6496–6500.
Warburg O, Posener K, Negelein E . Ueber den Stoffwechsel der Tumoren. Biochem Z 1924; 152: 319–344.
Kim JW, Tchernyshyov I, Semenza GL, Dang CV . HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 2006; 3: 177–185.
Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC . HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 2006; 3: 187–197.
Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL . HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 2007; 129: 111–122.
Ullah MS, Davies AJ, Halestrap AP . The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 2006; 281: 9030–9037.
Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL . HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 2006; 291: L941–L949.
Folkman J, Merler E, Abernathy C, Williams G . Isolation of a tumor factor responsible for angiogenesis. J Exp Med 1971; 133: 275–288.
Shweiki D, Itin A, Soffer D, Keshet E . Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992; 359: 843–845.
Ryan HE, Lo J, Johnson RS . HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 1998; 17: 3005–3015.
Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999; 286: 2511–2514.
Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS et al. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1alpha. Genes Dev 2001; 15: 2520–2532.
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996; 16: 4604–4613.
Gerber HP, Condorelli F, Park J, Ferrara N . Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem 1997; 272: 23659–23667.
Levy NS, Chung S, Furneaux H, Levy AP . Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem 1998; 273: 6417–6423.
Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP et al. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 2004; 6: 485–495.
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998; 394: 485–490.
Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res 2000; 60: 4010–4015.
Blouw B, Song H, Tihan T, Bosze J, Ferrara N, Gerber HP et al. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell 2003; 4: 133–146.
Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 2000; 157: 411–421.
Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 1999; 59: 5830–5835.
Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL . Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer 2000; 88: 2606–2618.
Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G . Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res 2000; 60: 4693–4696.
Elson DA, Ryan HE, Snow JW, Johnson R, Arbeit JM . Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res 2000; 60: 6189–6195.
Ivan M, Kaelin Jr WG . The von Hippel–Lindau tumor suppressor protein. Curr Opin Genet Dev 2001; 11: 27–34.
Pollard PJ, Spencer-Dene B, Shukla D, Howarth K, Nye E, El-Bahrawy M et al. Targeted inactivation of fh1 causes proliferative renal cyst development and activation of the hypoxia pathway. Cancer Cell 2007; 11: 311–319.
An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM . Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature 1998; 392: 405–408.
Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev 2000; 14: 391–396.
Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B . Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J 1998; 17: 5085–5094.
Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL . Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res 1999; 59: 3915–3918.
Richard DE, Berra E, Pouyssegur J . Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem 2000; 275: 26765–26771.
Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J . p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J Biol Chem 1999; 274: 32631–32637.
Moeller BJ, Cao Y, Li CY, Dewhirst MW . Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004; 5: 429–441.
Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q et al. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell 2007; 26: 63–74.
Liao D, Corle C, Seagroves TN, Johnson RS . Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res 2007; 67: 563–572.
Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuyama H, Baek JH et al. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel–Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res 2006; 66: 2725–2731.
Esteban MA, Tran MG, Harten SK, Hill P, Castellanos MC, Chandra A et al. Regulation of E-cadherin expression by VHL and hypoxia-inducible factor. Cancer Res 2006; 66: 3567–3575.
Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W . Chemokine receptor CXCR4 downregulated by von Hippel–Lindau tumour suppressor pVHL. Nature 2003; 425: 307–311.
Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 2006; 440: 1222–1226.
Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 1998; 12: 149–162.
Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC . Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 1997; 386: 403–407.
Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A et al. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci USA 1997; 94: 9102–9107.
Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H et al. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia–reperfusion injury. Circulation 2003; 108: 79–85.
Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR . Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci USA 2002; 99: 821–826.
Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 1999; 103: 691–696.
Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 2003; 112: 645–657.
Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V . Cutting edge: essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol 2007; 178: 7516–7519.
Huang Y, Hickey RP, Yeh JL, Liu D, Dadak A, Young LH et al. Cardiac myocyte-specific HIF-1alpha deletion alters vascularization, energy availability, calcium flux, and contractility in the normoxic heart. FASEB J 2004; 18: 1138–1140.
Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH . Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 2004; 114: 1098–1106.
Provot S, Zinyk D, Gunes Y, Kathri R, Le Q, Kronenberg HM et al. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol 2007; 177: 451–464.
Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 2007; 117: 1616–1626.
Warnecke C, Griethe W, Weidemann A, Jurgensen JS, Willam C, Bachmann S et al. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB J 2003; 17: 1186–1188.
Kim WY, Safran M, Buckley MR, Ebert BL, Glickman J, Bosenberg M et al. Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. EMBO J 2006; 25: 4650–4662.
Acknowledgements
AW was supported by a scholarship of the German Research Foundation (DFG WE4275/1-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by NS Chandel
Rights and permissions
About this article
Cite this article
Weidemann, A., Johnson, R. Biology of HIF-1α. Cell Death Differ 15, 621–627 (2008). https://doi.org/10.1038/cdd.2008.12
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2008.12
Keywords
This article is cited by
-
Enhancement of cardiac angiogenesis in a myocardial infarction rat model using selenium alone and in combination with PTXF: the role of Akt/HIF-1α signaling pathway
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Regulation of genes involved in the metabolic adaptation of murine microglial cells in response to elevated HIF-1α mediated activation
Immunogenetics (2024)
-
Analogies between the periphery of cancer and the leading edge of pulmonary fibrosis
Journal of Translational Medicine (2023)
-
Pharmacological inhibition of neuropeptide Y receptors Y1 and Y5 reduces hypoxic breast cancer migration, proliferation, and signaling
BMC Cancer (2023)
-
A microfluidic-based PDAC organoid system reveals the impact of hypoxia in response to treatment
Cell Death Discovery (2023)