Abstract
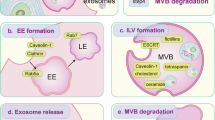
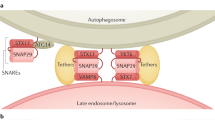
In the majority of cell types, multivesicular bodies (MVBs) are a special kind of late endosomes, crucial intermediates in the internalization of nutrients, ligands and receptors through the endolysosomal system. ESCRT-0, I, II and III (endosomal sorting complex required for transport) are involved in the sorting of proteins into MVBs, generating the intraluminal vesicles. Autophagy is a lysosomal degradation pathway for cytoplasmic components such as proteins and organelles. The autophagosome, a well-characterized structure of the autophagy pathway, can fuse with endocytic structures such as MVBs to generate the amphisome. Finally, the amphisome fuses with the lysosome to degrade the material wrapped inside. Currently, clear evidence suggests that efficient autophagic degradation requires functional MVBs. This review highlights the most recent advances in our understanding of the molecular machinery that participates in MVB biogenesis and regulates the interplay between autophagy and this organelle.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- MVB:
-
multivesicular body
- ESCRT:
-
endosomal sorting complex required for transport
- ConA:
-
concanavalin A
- WGA:
-
wheat germen agglutinin
- Hrs:
-
hepatocyte growth factor-regulated tyrosine kinase substrate
- Vps:
-
vacuolar sorting protein
- CHMP:
-
charged multivesicular body protein
- Tsg 101:
-
tumour susceptibility gene 101
- EEA1:
-
early endosome antigen 1
- M6P:
-
mannose 6-phosphate
- Atg:
-
autophagy related protein
- LC3:
-
microtubule-associated protein light chain 3
- UIM:
-
ubiquitin interaction motif
- UEV:
-
ubiquitin E2 variant
- DUB:
-
deubiquitinating enzyme
- PI3K:
-
phosphatidylinositol-3-kinase
- UVRAG:
-
UV radiation resistance associated gene
- Rab:
-
Ras-related protein in brain
- Bcl-2:
-
B-cell lymphoma protein 2
- SNARE:
-
N-ethylmaleimide sensitive protein attachment protein receptor
- SKD1:
-
suppressor of K+ uptake growth defect
- WM:
-
wortmanin
References
Mellman I . Membranes and sorting. Curr Opin Cell Biol 1996; 8: 497–498.
Harding C, Heuser J, Stahl P . Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 1983; 97: 329–339.
Harding C, Heuser J, Stahl P . Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol 1984; 35: 256–263.
Johnstone RM, Mathew A, Mason AB, Teng K . Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol 1991; 147: 27–36.
Johnstone RM . The Jeanne Manery-Fisher Memorial Lecture 1991. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem Cell Biol 1992; 70: 179–190.
Johnstone RM . Cleavage of the transferrin receptor by human granulocytes: differential proteolysis of the exosome-bound TFR. J Cell Physiol 1996; 168: 333–345.
Thery C, Zitvogel L, Amigorena S . Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–579.
Gronowicz G, Swift H, Steck TL . Maturation of the reticulocyte in vitro. J Cell Sci 1984; 71: 177–197.
Dunn Jr WA . Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J Cell Biol 1990; 110: 1935–1945.
Klionsky DJ, Emr SD . Autophagy as a regulated pathway of cellular degradation. Science 2000; 290: 1717–1721.
Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E . Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 2005; 1: 84–91.
Berg TO, Fengsrud M, Stromhaug PE, Berg T, Seglen PO . Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem 1998; 273: 21883–21892.
Dunn Jr WA . Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol 1994; 4: 139–143.
Gordon PB, Seglen PO . Prelysosomal convergence of autophagic and endocytic pathways. Biochem Biophys Res Commun 1988; 151: 40–47.
Gordon PB, Hoyvik H, Seglen PO . Prelysosomal and lysosomal connections between autophagy and endocytosis. Biochem J 1992; 283 (Part 2): 361–369.
Shintani T, Klionsky DJ . Autophagy in health and disease: a double-edged sword. Science 2004; 306: 990–995.
Eskelinen EL . Maturation of autophagic vacuoles in mammalian cells. Autophagy 2005; 1: 1–10.
Yi J, Tang XM . The convergent point of the endocytic and autophagic pathways in Leydig cells. Cell Res 1999; 9: 243–253.
Fader CM, Sanchez D, Furlan M, Colombo MI . Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic 2008; 9: 230–250.
Hershko A . Mechanisms and regulation of the degradation of cyclin B. Philos Trans R Soc Lond B Biol Sci 1999; 354: 1571–1575.
Hicke L, Dunn R . Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol 2003; 19: 141–172.
Katzmann DJ, Odorizzi G, Emr SD . Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol 2002; 3: 893–905.
Teo H, Veprintsev DB, Williams RL . Structural insights into endosomal sorting complex required for transport (ESCRT-I) recognition of ubiquitinated proteins. J Biol Chem 2004; 279: 28689–28696.
Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK et al. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 1989; 243: 1576–1583.
Thrower JS, Hoffman L, Rechsteiner M, Pickart CM . Recognition of the polyubiquitin proteolytic signal. EMBO J 2000; 19: 94–102.
Raiborg C, Rusten TE, Stenmark H . Protein sorting into multivesicular endosomes. Curr Opin Cell Biol 2003; 15: 446–455.
Babst M . A protein's final ESCRT. Traffic 2005; 6: 2–9.
Nickerson DP, Russell MR, Odorizzi G . A concentric circle model of multivesicular body cargo sorting. EMBO Rep 2007; 8: 644–650.
Saksena S, Sun J, Chu T, Emr SD . ESCRTing proteins in the endocytic pathway. Trends Biochem Sci 2007; 32: 561–573.
Williams RL, Urbe S . The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol 2007; 8: 355–368.
Raiborg C, Stenmark H . Hrs and endocytic sorting of ubiquitinated membrane proteins. Cell Struct Funct 2002; 27: 403–408.
Gaullier JM, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H, Aasland R . FYVE fingers bind PtdIns(3)P. Nature 1998; 394: 432–433.
Bache KG, Brech A, Mehlum A, Stenmark H . Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J Cell Biol 2003; 162: 435–442.
Katzmann DJ, Stefan CJ, Babst M, Emr SD . Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J Cell Biol 2003; 162: 413–423.
Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerod L, Fisher EM et al. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol 2007; 179: 485–500.
Azmi I, Davies B, Dimaano C, Payne J, Eckert D, Babst M et al. Recycling of ESCRTs by the AAA-ATPase Vps4 is regulated by a conserved VSL region in Vta1. J Cell Biol 2006; 172: 705–717.
Nickerson DP, West M, Odorizzi G . Did2 coordinates Vps4-mediated dissociation of ESCRT-III from endosomes. J Cell Biol 2006; 175: 715–720.
Lakkaraju A, Rodriguez-Boulan E . Cell biology: caught in the traffic. Nature 2007; 448: 266–267.
Sadoul R . Do Alix and ALG-2 really control endosomes for better or for worse? Biol Cell 2006; 98: 69–77.
Luhtala N, Odorizzi G . Bro1 coordinates deubiquitination in the multivesicular body pathway by recruiting Doa4 to endosomes. J Cell Biol 2004; 166: 717–729.
Nikko E, Marini AM, Andre B . Permease recycling and ubiquitination status reveal a particular role for Bro1 in the multivesicular body pathway. J Biol Chem 2003; 278: 50732–50743.
Odorizzi G, Katzmann DJ, Babst M, Audhya A, Emr SD . Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J Cell Sci 2003; 116: 1893–1903.
Reggiori F, Pelham HR . Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J 2001; 20: 5176–5186.
White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE . EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J 2006; 25: 1–12.
Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF et al. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell 2006; 10: 343–354.
Mizushima N, Ohsumi Y, Yoshimori T . Autophagosome formation in mammalian cells. Cell Struct Funct 2002; 27: 421–429.
Bohley P, Seglen PO . Proteases and proteolysis in the lysosome. Experientia 1992; 48: 151–157.
Klionsky DJ, Ohsumi Y . Vacuolar import of proteins and organelles from the cytoplasm. Annu Rev Cell Dev Biol 1999; 15: 1–32.
Levine B, Klionsky DJ . Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004; 6: 463–477.
Meijer AJ, Codogno P . Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol 2004; 36: 2445–2462.
Kimura S, Noda T, Yoshimori T . Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007; 3: 452–460.
Liou W, Geuze HJ, Geelen MJ, Slot JW . The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J Cell Biol 1997; 136: 61–70.
Lucocq J, Walker D . Evidence for fusion between multilamellar endosomes and autophagosomes in HeLa cells. Eur J Cell Biol 1997; 72: 307–313.
Punnonen EL, Autio S, Kaija H, Reunanen H . Autophagic vacuoles fuse with the prelysosomal compartment in cultured rat fibroblasts. Eur J Cell Biol 1993; 61: 54–66.
Tooze J, Hollinshead M, Ludwig T, Howell K, Hoflack B, Kern H . In exocrine pancreas, the basolateral endocytic pathway converges with the autophagic pathway immediately after the early endosome. J Cell Biol 1990; 111: 329–345.
Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K et al. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol 2001; 152: 657–668.
Klionsky DJ . The molecular machinery of autophagy: unanswered questions. J Cell Sci 2005; 118: 7–18.
Nemoto T, Tanida I, Tanida-Miyake E, Minematsu-Ikeguchi N, Yokota M, Ohsumi M et al. The mouse APG10 homologue, an E2-like enzyme for Apg12p conjugation, facilitates MAP-LC3 modification. J Biol Chem 2003; 278: 39517–39526.
Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y . The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J 2001; 20: 5971–5981.
Tanida I, Nishitani T, Nemoto T, Ueno T, Kominami E . Mammalian Apg12p, but not the Apg12p.Apg5p conjugate, facilitates LC3 processing. Biochem Biophys Res Commun 2002; 296: 1164–1170.
Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T et al. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem 2007; 282: 37298–37302.
Mortimore GE, Schworer CM . Induction of autophagy by amino-acid deprivation in perfused rat liver. Nature 1977; 270: 174–176.
Mortimore GE, Poso AR . Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu Rev Nutr 1987; 7: 539–564.
Blommaart EF, Luiken JJ, Blommaart PJ, Van Woerkom GM, Meijer AJ . Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem 1995; 270: 2320–2326.
Klionsky DJ, Emr SD . Autophagy as a regulated pathway of cellular degradation [in process citation]. Science 2000; 290: 1717–1721.
Seglen PO, Gordon PB, Poli A . Amino acid inhibition of the autophagic/lysosomal pathway of protein degradation in isolated rat hepatocytes. Biochim Biophys Acta 1980; 630: 103–118.
Van Sluijters DA, Dubbelhuis PF, Blommaart EF, Meijer AJ . Amino-acid-dependent signal transduction [in process citation]. Biochem J 2000; 351 (Part 3): 545–550.
Eskelinen EL, Prescott AR, Cooper J, Brachmann SM, Wang L, Tang X et al. Inhibition of autophagy in mitotic animal cells. Traffic 2002; 3: 878–893.
Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P . Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem 2000; 275: 992–998.
Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ . The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem 1997; 243: 240–246.
Takano-Ohmuro H, Mukaida M, Kominami E, Morioka K . Autophagy in embryonic erythroid cells: its role in maturation. Eur J Cell Biol 2000; 79: 759–764.
Suzuki K, Kubota Y, Sekito T, Ohsumi Y . Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells 2007; 12: 209–218.
Suzuki K, Ohsumi Y . Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS Lett 2007; 581: 2156–2161.
Kihara A, Noda T, Ishihara N, Ohsumi Y . Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol 2001; 152: 519–530.
Liang C, Sir D, Lee S, Ou JH, Jung JU . Beyond autophagy: the role of UVRAG in membrane trafficking. Autophagy 2008; 4: 817–820.
Gutierrez MG, Munafo DB, Beron W, Colombo MI . Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci 2004; 117: 2687–2697.
Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P et al. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 2004; 117: 4837–4848.
Ravikumar B, Imarisio S, Sarkar S, O'Kane CJ, Rubinsztein DC . Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J Cell Sci 2008; 121: 1649–1660.
Ding WX, Yin XM . Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy 2008; 4: 141–150.
Hoyer-Hansen M, Jaattela M . AMP-activated protein kinase: a universal regulator of autophagy? Autophagy 2007; 3: 381–383.
Gordon PB, Holen I, Fosse M, Rotnes JS, Seglen PO . Dependence of hepatocytic autophagy on intracellularly sequestered calcium. J Biol Chem 1993; 268: 26107–26112.
Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell 2007; 25: 193–205.
Hoyer-Hansen M, Jaattela M . Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 2007; 14: 1576–1582.
Savina A, Furlan M, Vidal M, Colombo MI . Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem 2003; 278: 20083–20090.
Savina A, Fader CM, Damiani MT, Colombo MI . Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 2005; 6: 131–143.
Abeliovich H, Zhang C, Dunn Jr WA, Shokat KM, Klionsky DJ . Chemical genetic analysis of Apg1 reveals a non-kinase role in the induction of autophagy. Mol Biol Cell 2003; 14: 477–490.
Fass E, Shvets E, Degani I, Hirschberg K, Elazar Z . Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem 2006; 281: 36303–36316.
Kochl R, Hu XW, Chan EY, Tooze SA . Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic 2006; 7: 129–145.
Nara A, Mizushima N, Yamamoto A, Kabeya Y, Ohsumi Y, Yoshimori T . SKD1 AAA ATPase-dependent endosomal transport is involved in autolysosome formation. Cell Struct Funct 2002; 27: 29–37.
Fader CM, Colombo MI . Multivesicular bodies and autophagy in erythrocyte maturation. Autophagy 2006; 2: 122–125.
Zeng X, Overmeyer JH, Maltese WA . Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci 2006; 119: 259–270.
Iwata A, Riley BE, Johnston JA, Kopito RR . HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem 2005; 280: 40282–40292.
Iwata J, Ezaki J, Komatsu M, Yokota S, Ueno T, Tanida I et al. Excess peroxisomes are degraded by autophagic machinery in mammals. J Biol Chem 2006; 281: 4035–4041.
Ravikumar B, Rubinsztein DC . Can autophagy protect against neurodegeneration caused by aggregate-prone proteins? NeuroReport 2004; 15: 2443–2445.
Yamamoto A, Cremona ML, Rothman JE . Autophagy-mediated clearance of huntingtin aggregates triggered by the insulin-signaling pathway. J Cell Biol 2006; 172: 719–731.
Parkinson N, Ince PG, Smith MO, Highley R, Skibinski G, Andersen PM et al. ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 2006; 67: 1074–1077.
Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet 2005; 37: 806–808.
Lee JA, Beigneux A, Ahmad ST, Young SG, Gao FB . ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr Biol 2007; 17: 1561–1567.
Rusten TE, Filimonenko M, Rodahl LM, Stenmark H, Simonsen A . ESCRTing autophagic clearance of aggregating proteins. Autophagy 2007; 4: 1166–1172.
Philips JA, Porto MC, Wang H, Rubin EJ, Perrimon N . ESCRT factors restrict mycobacterial growth. Proc Natl Acad Sci USA 2008; 105: 3070–3075.
Rusten TE, Simonsen A . ESCRT functions in autophagy and associated disease. Cell Cycle 2008; 7: 1166–1172.
Acknowledgements
Our study in this area is funded by grants from Agencia Nacional de Promoción Científica y Tecnológica (PICT2004 no. 20711 and PICT 2005 no. 38420), CONICET (PIP no. 5943) and SECTyP (Universidad Nacional de Cuyo).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Melino
Rights and permissions
About this article
Cite this article
Fader, C., Colombo, M. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ 16, 70–78 (2009). https://doi.org/10.1038/cdd.2008.168
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2008.168
Keywords
This article is cited by
-
Endosomal sorting results in a selective separation of the protein corona from nanoparticles
Nature Communications (2023)
-
Induction of lysosomal exocytosis and biogenesis via TRPML1 activation for the treatment of uranium-induced nephrotoxicity
Nature Communications (2023)
-
The S100A10–AnxA2 complex is associated with the exocytosis of hepatitis B virus in intrauterine infection
Laboratory Investigation (2022)
-
In human astrocytes neurotropic flaviviruses increase autophagy, yet their replication is autophagy-independent
Cellular and Molecular Life Sciences (2022)
-
SKP-SCs transplantation alleviates 6-OHDA-induced dopaminergic neuronal injury by modulating autophagy
Cell Death & Disease (2021)