Abstract
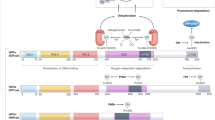
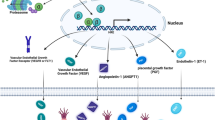
The transcriptional response to hypoxia is primarily mediated by two hypoxia-inducible factors – HIF-1α and HIF-2α. While these proteins are highly homologous, increasing evidence suggests they have unique transcriptional targets and differential impact on tumor growth. Furthermore, non-transcriptional effects of the HIF-α subunits, including effects on the Notch and c-Myc pathways, contribute to their distinct functions. HIF-2α transcriptional targets include genes involved in erythropoiesis, angiogenesis, metastasis, and proliferation. Therefore, HIF-2α contributes significantly to both normal physiology as well as tumorigenesis. Here, we summarize the function of HIF-2α during development as well as its contribution to pathologic conditions, such as tumors and vascular disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ARNT:
-
aryl hydrocarbon receptor nuclear translocator
- Epo:
-
erythropoietin
- HIFs:
-
hypoxia-inducible factors
- HRE:
-
hypoxia response element
- IRE:
-
iron response element
- IRP:
-
iron regulatory protein
- LOX:
-
lysyl oxidase
- PAS:
-
Per-ARNT-Sim
- pVHL:
-
von Hippel–Lindau
- RCC:
-
renal clear cell carcinoma
- TGF-α:
-
transforming growth factor-α
- TAD:
-
transcriptional activation domain
- UTR:
-
untranslated region
- VEGF:
-
vascular endothelial growth factor
References
Harris AL . Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47.
Covello KL, Simon MC . HIFs, hypoxia, and vascular development. Curr Top Dev Biol 2004; 62: 37–54.
Jain RK . Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005; 307: 58–62.
Gu YZ, Hogenesch JB, Bradfield CA . The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol 2000; 40: 519–561.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ et al. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001; 292: 468–472.
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR et al. C.elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001; 107: 43–54.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999; 399: 271–275.
Yu F, White SB, Zhao Q, Lee FS . HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci USA 2001; 98: 9630–9635.
Bruick RK, McKnight SL . A conserved family of prolyl-4-hydroxylases that modify HIF. Science 2001; 294: 1337–1340.
Wenger RH, Stiehl DP, Camenisch G . Integration of oxygen signaling at the consensus HRE. Sci STKE 2005; 2005: re12.
Tian H, Mcknight SL, Russell DW . Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 1997; 11: 72–82.
Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y . A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA 1997; 94: 4273–4278.
Flamme I, Frohlich T, von Reutern M, Kappel A, Damert A, Risau W . HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1 alpha and developmentally expressed in blood vessels. Mech Dev 1997; 63: 51–60.
Hogenesch JB, Chan WK, Jackiw VH, Brown RC, Gu YZ, Pray-Grant M et al. Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem 1997; 272: 8581–8593.
Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL . The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev 1998; 12: 3320–3324.
Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J 2003; 17: 271–273.
Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 2006; 10: 413–423.
Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL et al. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood 1998; 92: 2260–2268.
Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 2002; 8: 702–710.
Jurgensen JS, Rosenberger C, Wiesener MS, Warnecke C, Horstrup JH, Grafe M et al. Persistent induction of HIF-1alpha and -2alpha in cardiomyocytes and stromal cells of ischemic myocardium. FASEB J 2004; 18: 1415–1417.
Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC . Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 2003; 23: 9361–9374.
Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M et al. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J 2004; 18: 1462–1464.
Wang V, Davis DA, Haque M, Huang LE, Yarchoan R . Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res 2005; 65: 3299–3306.
Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel–Lindau-associated renal cell carcinoma. Mol Cell Biol 2005; 25: 5675–5686.
Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC . Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol Cell Biol 2006; 26: 3514–3526.
Grabmaier K, de Weijert MCA, Verhaegh GW, Schalken JA, Oosterwijk E . Strict regulation of CAIX(G250/MN) by HIF-1alpha in clear cell renal cell carcinoma. Oncogene 2004; 23: 5624–5631.
Baba M, Hirai S, Yamada-Okabe H, Hamada K, Tabuchi H, Kobayashi K et al. Loss of von Hippel–Lindau protein causes cell density dependent deregulation of CyclinD1 expression through hypoxia-inducible factor. Oncogene 2003; 22: 2728–2738.
Gunaratnam L, Morley M, Franovic A, de Paulsen N, Mekhail K, Parolin DA et al. Hypoxia inducible factor activates the transforming growth factor-alpha/epidermal growth factor receptor growth stimulatory pathway in VHL(−/−) renal cell carcinoma cells. J Biol Chem 2003; 278: 44966–44974.
Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev 2006; 20: 557–570.
Gort EH, van Haaften G, Verlaan I, Groot AJ, Plasterk RH, Shvarts A et al. The TWIST1 oncogene is a direct target of hypoxia-inducible factor-2alpha. Oncogene 2007 [e-pub ahead of print].
Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC . Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci USA 2007; 104: 2301–2306.
O'Rourke JF, Tian YM, Ratcliffe PJ, Pugh CW . Oxygen-regulated and transactivating domains in endothelial PAS protein 1: comparison with hypoxia-inducible factor-1alpha. J Biol Chem 1999; 274: 2060–2071.
Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK . FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 2002; 16: 1466–1471.
Mahon PC, Hirota K, Semenza GL . FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 2001; 15: 2675–2686.
Lau KW, Tian YM, Raval RR, Ratcliffe PJ, Pugh CW . Target gene selectivity of hypoxia-inducible factor-alpha in renal cancer cells is conveyed by post-DNA-binding mechanisms. Br J Cancer 2007; 96: 1284–1292.
Hu CJ, Sataur A, Wang L, Chen H, Simon MC . The N-terminal transactivation domain confers target gene specificity of hypoxia inducible factors HIF-1{alpha} and HIF-2. Mol Biol Cell 2007; 18: 4528–4542.
Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T et al. Cooperative interaction of hypoxia-inducible factor-2alpha (HIF-2alpha) and Ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (Flk-1). J Biol Chem 2003; 278: 7520–7530.
Aprelikova O, Wood M, Tackett S, Chandramouli GV, Barrett JC . Role of ETS transcription factors in the hypoxia-inducible factor-2 target gene selection. Cancer Res 2006; 66: 5641–5647.
Peng J, Zhang L, Drysdale L, Fong GH . The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl Acad Sci USA 2000; 97: 8386–8391.
Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet 2003; 35: 331–340.
Norris ML, Millhorn DE . Hypoxia-induced protein binding to O2-responsive sequences on the tyrosine hydroxylase gene. J Biol Chem 1995; 270: 23774–23779.
Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D et al. Heterozygous deficiency of hypoxia-inducible factor-2alpha protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest 2003; 111: 1519–1527.
Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA . The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood 2003; 102: 1634–1640.
Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M et al. HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood 2005; 105: 3133–3140.
Covello KL, Simon MC, Keith B . Targeted replacement of hypoxia-inducible factor-1alpha by a hypoxia-inducible factor-2alpha knock-in allele promotes tumor growth. Cancer Res 2005; 65: 2277–2286.
Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 2007; 117: 1068–1077.
Chavez JC, Baranova O, Lin J, Pichiule P . The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci 2006; 26: 9471–9481.
Rolfs A, Kvietikova I, Gassmann M, Wenger RH . Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem 1997; 272: 20055–20062.
Hentze MW, Muckenthaler MU, Andrews NC . Balancing acts: molecular control of mammalian iron metabolism. Cell 2004; 117: 285–297.
Sanchez M, Galy B, Muckenthaler MU, Hentze MW . Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat Struct Mol Biol 2007; 14: 420–426.
Kaelin Jr WG . Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer 2002; 2: 673–682.
Kim WY, Kaelin WG . Role of VHL gene mutation in human cancer. J Clin Oncol 2004; 22: 4991–5004.
Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM et al. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 2002; 1: 459–468.
Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD . The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell 2002; 1: 247–255.
Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin Jr WG . Inhibition of HIF is necessary for tumor suppression by the von Hippel–Lindau protein. Cancer Cell 2002; 1: 237–246.
Kondo K, Kim WY, Lechpammer M, Kaelin Jr WG . Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol 2003; 1: E83.
Li L, Zhang L, Zhang X, Yan Q, Minamishima YA, Olumi AF et al. Hypoxia-inducible factor linked to differential kidney cancer risk seen with type 2A and type 2B VHL mutations. Mol Cell Biol 2007; 27: 5381–5392.
Rathmell WK, Hickey MM, Bezman NA, Chmielecki CA, Carraway NC, Simon MC . In vitro and in vivo models analyzing von Hippel–Lindau disease-specific mutations. Cancer Res 2004; 64: 8595–8603.
Hickey M, Rathmell WK, Lam JC, Bezman NA, Simon MC . von Hippel–Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2a signaling and splenic erythropoiesis. J Clin Invest 2007; 117: 3879–3889.
Liu MY, Poellinger L, Walker CL . Up-regulation of hypoxia-inducible factor 2alpha in renal cell carcinoma associated with loss of Tsc-2 tumor suppressor gene. Cancer Res 2003; 63: 2675–2680.
Smith K, Gunaratnam L, Morley M, Franovic A, Mekhail K, Lee S . Silencing of epidermal growth factor receptor suppresses hypoxia-inducible factor-2-driven VHL−/− renal cancer. Cancer Res 2005; 65: 5221–5230.
de Paulsen N, Brychzy A, Fournier MC, Klausner RD, Gnarra JR, Pause A et al. Role of transforming growth factor-alpha in von Hippel–Lindau (VHL)(−/−) clear cell renal carcinoma cell proliferation: a possible mechanism coupling VHL tumor suppressor inactivation and tumorigenesis. Proc Natl Acad Sci USA 2001; 98: 1387–1392.
Yan Q, Bartz S, Mao M, Li L, Kaelin Jr WG . The hypoxia-inducible factor 2alpha N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol Cell Biol 2007; 27: 2092–2102.
Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE . HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J 2004; 23: 1949–1956.
Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC . HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 2007; 11: 335–347.
Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 2007; 11: 407–420.
Corn PG, Ricci MS, Scata KA, Arsham AM, Simon MC, Dicker DT et al. Mxi1 is induced by hypoxia in a HIF-1-dependent manner and protects cells from c-Myc-induced apoptosis. Cancer Biol Ther 2005; 4: 1285–1294.
Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell 2005; 9: 617–628.
Condeelis J, Pollard JW . Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006; 124: 263–266.
Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 2000; 157: 411–421.
Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS et al. Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in human breast cancer. Cancer Res 2002; 62: 1326–1329.
Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W . Chemokine receptor CXCR4 downregulated by von Hippel–Lindau tumour suppressor pVHL. Nature 2003; 425: 307–311.
Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 2006; 440: 1222–1226.
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004; 117: 927–939.
Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA 2002; 99: 7021–7026.
Acker T, Diez-Juan A, Aragones J, Tjwa M, Brusselmans K, Moons L et al. Genetic evidence for a tumor suppressor role of HIF-2alpha. Cancer Cell 2005; 8: 131–141.
Brusselmans K, Bono F, Maxwell P, Dor Y, Dewerchin M, Collen D et al. Hypoxia-inducible factor-2alpha (HIF-2alpha) is involved in the apoptotic response to hypoglycemia but not to hypoxia. J Biol Chem 2001; 276: 39192–39196.
Gordan JD, Thompson CB, Simon MC . HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 2007; 12: 108–113.
Mack FA, Rathmell WK, Arsham AM, Gnarra J, Keith B, Simon MC . Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth. Cancer Cell 2003; 3: 75–88.
Mack FA, Patel JH, Biju MP, Haase VH, Simon MC . Decreased growth of Vhl−/− fibrosarcomas is associated with elevated levels of cyclin kinase inhibitors p21 and p27. Mol Cell Biol 2005; 25: 4565–4578.
Rankin EB, Tomaszewski JE, Haase VH . Renal cyst development in mice with conditional inactivation of the von Hippel–Lindau tumor suppressor. Cancer Res 2006; 66: 2576–2583.
Melillo G . Targeting hypoxia cell signaling for cancer therapy. Cancer Metastasis Rev 2007; 26: 341–352.
Carroll VA, Ashcroft M . Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel–Lindau function: implications for targeting the HIF pathway. Cancer Res 2006; 66: 6264–6270.
Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell 2004; 6: 33–43.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by RA Knight
Rights and permissions
About this article
Cite this article
Patel, S., Simon, M. Biology of hypoxia-inducible factor-2α in development and disease. Cell Death Differ 15, 628–634 (2008). https://doi.org/10.1038/cdd.2008.17
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2008.17
Keywords
This article is cited by
-
Pharmacological inhibition of neuropeptide Y receptors Y1 and Y5 reduces hypoxic breast cancer migration, proliferation, and signaling
BMC Cancer (2023)
-
Staufen1 controls mitochondrial metabolism via HIF2α in embryonal rhabdomyosarcoma and promotes tumorigenesis
Cellular and Molecular Life Sciences (2023)
-
Discovery of novel direct small-molecule inhibitors targeting HIF-2α using structure-based virtual screening, molecular dynamics simulation, and MM-GBSA calculations
Molecular Diversity (2023)
-
Unveiling OASIS family as a key player in hypoxia–ischemia cases induced by cocaine using generative adversarial networks
Scientific Reports (2022)
-
Genomic analysis of hypoxia inducible factor alpha in ray-finned fishes reveals missing Ohnologs and evidence of widespread positive selection
Scientific Reports (2022)