Abstract
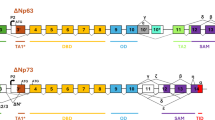
Regulation of the p73 gene is complex due to the presence of two promoters and the very complex mRNA maturation in both the N-terminal and C-terminal parts of the protein. We have found an additional regulation mechanism for the p73-α form that occurs through proteolytic cleavage connected to the activity of the serine protease HtrA2. Following apoptotic stimuli, HtrA2 accumulates in the nucleus and cleaves p73α in the C-terminal portion, enabling the protein to increase its transactivation activity on the apoptotic gene bax but not on the cell-cycle regulator gene p21. In the presence of HtrA2, p73 is more prone to cause caspase activation and nuclei fragmentation: p73 needs HtrA2 to activate and enhance its apoptotic functions. This new relation between p73 and HtrA2 may help to understand the different behavior of the p73 protein in cell physiology and in the responses of cancer cells to chemotherapy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- TA:
-
transactivation proficient
- DN:
-
N-terminus deficient
- HtrA2:
-
high temperature requirement A
- MEF:
-
mouse embryonic fibroblast
- P1:
-
TA promoter
- P2:
-
DN promoter
- doxo:
-
doxorubicin
References
Moll UM, Erster S, Zaika A . p53, p63 and p73 – solos, alliances and feuds among family members. Biochim Biophys Acta 2001; 1552: 47–59.
Melino G, De Laurenzi V, Vousden KH . p73: friend or foe in tumorigenesis. Nat Rev Cancer 2002; 2: 605–615.
Zhu J, Jiang J, Zhou W, Chen X . The potential tumor suppressor p73 differentially regulates cellular p53 target genes. Cancer Res 1998; 58: 5061–5065.
Jost CA, Marin MC, Kaelin Jr WG . p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature 1997; 389: 191–194.
Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell 1997; 90: 809–819.
Maisse C, Guerrieri P, Melino G . p73 and p63 protein stability: the way to regulate function? Biochem Pharmacol 2003; 66: 1555–1561.
Ishimoto O, Kawahara C, Enjo K, Obinata M, Nukiwa T, Ikawa S . Possible oncogenic potential of deltaNp73: a newly identified isoform of human p73. Cancer Res 2002; 62: 636–641.
Putzer BM, Tuve S, Tannapfel A, Stiewe T . Increased deltaN-p73 expression in tumors by upregulation of the E2F1-regulated, TA-promoter-derived deltaN′-p73 transcript. Cell Death Differ 2003; 10: 612–614.
Nakagawa T, Takahashi M, Ozaki T, Watanabe KK, Todo S, Mizuguchi H et al. Autoinhibitory regulation of p73 by delta Np73 to modulate cell survival and death through a p73-specific target element within the delta Np73 promoter. Mol Cell Biol 2002; 22: 2575–2585.
Zaika AI, Slade N, Erster SH, Sansome C, Joseph TW, Pearl M et al. DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J Exp Med 2002; 196: 765–780.
Vossio S, Palescandolo E, Pediconi N, Moretti F, Balsano C, Levrero M et al. DN-p73 is activated after DNA damage in a p53-dependent manner to regulate p53-induced cell cycle arrest. Oncogene 2002; 21: 3796–3803.
Grob TJ, Novak U, Maisse C, Barcaroli D, Luthi AU, Pirnia F et al. Human delta Np73 regulates a dominant negative feedback loop for TAp73 and p53. Cell Death Differ 2001; 8: 1213–1223.
De Laurenzi V, Costanzo A, Barcaroli D, Terrinoni A, Falco M, Annicchiarico-Petruzzelli M et al. Two new p73 splice variants, gamma and delta, with different transcriptional activity. J Exp Med 1998; 188: 1763–1768.
Concin N, Becker K, Slade N, Erster S, Mueller-Holzner E, Ulmer H et al. Transdominant deltaTAp73 isoforms are frequently up-regulated in ovarian cancer. Evidence for their role as epigenetic p53 inhibitors in vivo. Cancer Res 2004; 64: 2449–2460.
Slade N, Zaika AI, Erster S, Moll UM . DeltaNp73 stabilises TAp73 proteins but compromises their function due to inhibitory hetero-oligomer formation. Cell Death Differ 2004; 11: 357–360.
Hegde R, Srinivasula SM, Zhang Z, Wassell R, Mukattash R, Cilenti L et al. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein–caspase interaction. J Biol Chem 2002; 277: 432–438.
Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R . A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell 2001; 8: 613–621.
Verhagen AM, Silke J, Ekert PG, Pakusch M, Kaufmann H, Connolly LM et al. HtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J Biol Chem 2002; 277: 445–454.
Jin S, Kalkum M, Overholtzer M, Stoffel A, Chait BT, Levine AJ . CIAP1 and the serine protease HTRA2 are involved in a novel p53-dependent apoptosis pathway in mammals. Genes Dev 2003; 17: 359–367.
Kuninaka S, Iida SI, Hara T, Nomura M, Naoe H, Morisaki T et al. Serine protease Omi/HtrA2 targets WARTS kinase to control cell proliferation. Oncogene 2006; 26: 2395–2406.
Marabese M, Vikhanskaya F, Broggini M . p73: a chiaroscuro gene in cancer. Eur J Cancer 2007; 43: 1361–1372.
Zaika AI, Slade N, Erster SH, Sansome C, Joseph TW, Pearl M et al. DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J Exp Med 2002; 196: 765–780.
Yang QH, Church-Hajduk R, Ren J, Newild-typeon ML, Du C . Omi/HtrA2 catalytic cleavage of inhibitor of apoptosis (IAP) irreversibly inactivates IAPs and facilitates caspase activity in apoptosis. Genes Dev 2003; 17: 1487–1496.
Cilenti L, Soundarapandian MM, Kyriazis GA, Stratico V, Singh S, Gupta S et al. Regulation of HAX-1 anti-apoptotic protein by Omi/HtrA2 protease during cell death. J Biol Chem 2004; 279: 50295–50301.
Trencia A, Fiory F, Maitan MA, Vito P, Barbagallo AP, Perfetti A et al. Omi/HtrA2 promotes cell death by binding and degrading the anti-apoptotic protein ped/pea-15. J Biol Chem 2004; 279: 46566–46572.
Marabese M, Vikhanskaya F, Rainelli C, Sakai T, Broggini M . DNA damage induces transcriptional activation of p73 by removing C-EBPalpha repression on E2F1. Nucleic Acids Res 2003; 31: 6624–6632.
Acknowledgements
We thank V Fedele for special advices on HtrA2 experiments. We thank V De Laurenzi, G Melino, K Sabapathy and LM Martins for reagents. JD Baggott kindly revised the paper. The generous contribution of the Italian Association for Cancer Research is gratefully acknowledged. This work was partially supported by grants from the Italian Ministry of Health, Fondazione CARIPLO and Negri-Weizmann to MB. FV is a visiting scientist from the Institute of Cytology, Russian Academy of Science, St Petersburg, Russia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by KH Vousden
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Marabese, M., Mazzoletti, M., Vikhanskaya, F. et al. HtrA2 enhances the apoptotic functions of p73 on bax. Cell Death Differ 15, 849–858 (2008). https://doi.org/10.1038/cdd.2008.7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2008.7
Keywords
This article is cited by
-
Protease-independent control of parthanatos by HtrA2/Omi
Cellular and Molecular Life Sciences (2023)
-
∆Np73beta induces caveolin-1 in human non-small cell lung cancer cell line H1299
Tumor Biology (2016)
-
Protease Omi facilitates neurite outgrowth in mouse neuroblastoma N2a cells by cleaving transcription factor E2F1
Acta Pharmacologica Sinica (2015)
-
Role of the nucleus in apoptosis: signaling and execution
Cellular and Molecular Life Sciences (2015)