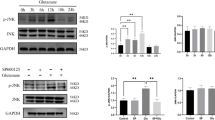
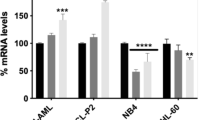
Abstract
A role for tissue transglutaminase (TG2) and its substrate dual leucine zipper-bearing kinase (DLK), an upstream component of the c-Jun N-terminal kinase (JNK) signaling pathway, has been previously suggested in the apoptotic response induced by calphostin C. In the current study, we directly tested this hypothesis by examining via pharmacological and RNA-interference approaches whether inhibition of expression or activity of TG2, DLK and JNK in mouse NIH 3T3 fibroblasts and human MDA-MB-231 breast cancer epithelial cells affects calphostin C-induced apoptosis. Our experiments with the selective JNK inhibitor SP600125 reveal that calphostin C is capable of causing JNK activation and JNK-dependent apoptosis in both cell lines. Small interfering RNA-mediated depletion of TG2 alone strongly reduces calphostin C action on JNK activity and apoptosis. Consistent with an active role for DLK in this cascade of event, cells deficient in DLK demonstrate a substantial delay of JNK activation and poly-ADP-ribose polymerase (PARP) cleavage in response to calphostin C, whereas overexpression of a recombinant DLK resistant to silencing, but sensitive to TG2-mediated oligomerization, reverses this effect. Importantly, combined depletion of TG2 and DLK further alters calphostin C effects on JNK activity, Bax translocation, caspase-3 activation, PARP cleavage and cell viability, demonstrating an obligatory role for TG2 and DLK in calphostin C-induced apoptosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- TG2:
-
tissue transglutaminase
- DLK:
-
dual leucine zipper-bearing kinase
- JNK:
-
c-Jun amino-terminal kinase
- PARP:
-
poly-ADP-ribose polymerase
References
Kobayashi E, Nakano H, Morimoto M, Tamaoki T . Calphostin C (UNC-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 1989; 159: 548–553.
Ikemoto H, Tani E, Matsumoto T, Nakano A, Furuyama J . Apoptosis of human glioma cells in response to calphostin C, a specific protein kinase C inhibitor. J Neurosurg 1995; 83: 1008–1016.
Zhu DM, Narla RK, Fang WH, Chia NC, Uckun FM . Calphostin C triggers calcium-dependent apoptosis in human acute lymphoblastic leukemia cells. Clin Cancer Res 1998; 4: 2967–2976.
Ozaki I, Tani E, Ikemoto H, Kitagawa H, Fujikawa H . Activation of stress-activated protein kinase/c-Jun NH2-terminal kinase and p38 kinase in calphostin C-induced apoptosis requires caspase-3-like proteases but is dispensable for cell death. J Biol Chem 1999; 274: 5310–5317.
Ikemoto H, Tani E, Ozaki I, Kitagawa H, Arita N . Calphostin C-mediated translocation and integration of Bax into mitochondria induces cytochrome c release before mitochondrial dysfunction. Cell Death Differ 2000; 7: 511–520.
Zheng XL, Gui Y, Du G, Frohman MA, Peng DQ . Calphostin-C induction of vascular smooth muscle cell apoptosis proceeds through phospholipase D and microtubule inhibition. J Biol Chem 2004; 279: 7112–7118.
Oliverio S, Amendola A, Di Sano F, Farrace MG, Fesus L, Nemes Z et al. Tissue transglutaminase-dependent posttranslational modification of the retinoblastoma gene product in promonocytic cells undergoing apoptosis. Mol Cell Biol 1997; 17: 6040–6048.
Hébert SS, Daviau A, Grondin G, Latreille M, Aubin RA, Blouin R . The mixed lineage kinase DLK is oligomerized by tissue transglutaminase during apoptosis. J Biol Chem 2000; 275: 32482–32490.
Robitaille K, Daviau A, Tucholski J, Johnson GV, Rancourt C, Blouin R . Tissue transglutaminase triggers oligomerization and activation of dual leucine zipper-bearing kinase in calphostin C-treated cells to facilitate apoptosis. Cell Death Differ 2004; 11: 542–549.
Lorand L, Graham RM . Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 2003; 4: 140–156.
Esposito C, Caputo I . Mammalian transglutaminases. Identification of substrates as a key to physiological function and physiopathological relevance. FEBS J 2005; 272: 615–631.
Fésüs L, Szondy Z . Transglutaminase 2 in the balance of cell death and survival. FEBS Lett 2005; 579: 3297–3302.
Tucholski J, Johnson GVW . Tissue transglutaminase differentially modulates apoptosis in a stimuli-dependent manner. J Neurochem 2002; 81: 780–791.
Antonyak MA, Singh US, Lee DA, Boehm JE, Combs C, Zgola MM et al. Effects of tissue transglutaminase on retinoic acid-induced cellular differentiation and protection against apoptosis. J Biol Chem 2001; 276: 33582–33587.
Antonyak MA, Boehm JE, Cerione RA . Phosphoinositide 3-kinase activity is required for retinoic acid-induced expression and activation of the tissue transglutaminase. J Biol Chem 2002; 277: 14712–14716.
Nemes Jr Z, Adány R, Balázs M, Boross P, Fésüs L . Identification of cytoplasmic actin as an abundant glutaminyl substrate for tissue transglutaminase in HL-60 and U937 cells undergoing apoptosis. J Biol Chem 1997; 272: 20577–20583.
Ballestar E, Abad C, Franco L . Core histones are glutaminyl substrates for tissue transglutaminase. J Biol Chem 1996; 271: 18817–18824.
Davis RJ . Signal transduction by the JNK group of MAP kinases. Cell 2000; 103: 239–252.
Harper SJ, LoGrasso P . Signalling for survival and death in neurones—The role of stress-activated kinases, JNK and P38. Cell Signalling 2001; 13: 299–310.
Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 1997; 389: 865–870.
Troy CM, Rabacchi SA, Xu Z, Maroney AC, Connors TJ, Shelanski ML et al. beta-Amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem 2001; 77: 157–164.
Maroney AC, Finn JP, Bozyczko-Coyne D, O’Kane TM, Neff NT, Tolkovsky AM et al. CEP-1347 (KT7515), an inhibitor of JNK activation, rescues sympathetic neurons and neuronally differentiated PC12 cells from death evoked by three distinct insults. J Neurochem 1999; 73: 1901–1912.
Mayne GC, Murray AW . Evidence that protein kinase C epsilon mediates phorbol ester inhibition of calphostin C- and tumor necrosis factor-alpha-induced apoptosis in U937 histiocytic lymphoma cells. J Biol Chem 1998; 273: 24115–24121.
Pollack IF, Kawecki S . The effect of calphostin C, a potent photodependent protein kinase C inhibitor, on the proliferation of glioma cells in vitro. J Neurooncol 1997; 31: 255–266.
Porter AG, Jänicke RU . Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999; 6: 99–104.
Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC . Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 1994; 371: 346–347.
Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 2001; 98: 13681–13686.
Kim BJ, Ryu SW, Song BJ . JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem 2006; 281: 21256–21265.
Mehta K, Fok J, Miller FR, Koul D, Sahin AA . Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res 2004; 10: 8068–8076.
Weston CR, Davis RJ . The JNK signal transduction pathway. Curr Opin Genet Dev 2002; 12: 14–21.
Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 2000; 288: 870–874.
Shin DM, Jeon JH, Kim CW, Cho SY, Kwon JC, Lee HJ et al. Cell type-specific activation of intracellular transglutaminase 2 by oxidative stress or ultraviolet irradiation: implications of transglutaminase 2 in age-related cataractogenesis. J Biol Chem 2004; 279: 15032–15039.
Fan G, Merritt SE, Kortenjann M, Shaw PE, Holzman LB . Dual leucine zipper-bearing kinase (DLK) activates p46SAPK and p38mapk but not ERK2. J Biol Chem 1996; 271: 24788–24793.
Gallo KA, Johnson GL . Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 2002; 3: 663–672.
Johnson GV, Cox TM, Lockhart JP, Zinnerman MD, Miller ML, Powers RE . Transglutaminase activity is increased in Alzheimer's disease brain. Brain Res 1997; 751: 323–329.
Rasmussen LK, Sorensen ES, Petersen TE, Gliemann J, Jensen PH . Identification of glutamine and lysine residues in Alzheimer amyloid β A4 peptide responsible for transglutaminase-catalysed homopolymerization and cross-linking to alpha 2 M receptor. FEBS Lett 1994; 338: 161–166.
Tucholski J, Kuret J, Johnson GV . Tau is modified by tissue transglutaminase in situ: possible functional and metabolic effects of polyamination. J Neurochem 1999; 73: 1871–1880.
Chen JSK, Konopleva M, Andreeff M, Multani AS, Pathak S, Mehta K . Drug-resistant breast carcinoma (MCF-7) cells are paradoxically sensitive to apoptosis. J Cell Physiol 2004; 200: 223–234.
Fok JY, Mehta K . Tissue transglutaminase induces the release of apoptosis inducing factor and results in apoptotic death of pancreatic cells. Apoptosis 2007; 12: 1455–1463.
Douziech M, Laberge G, Grondin G, Daigle N, Blouin R . Localization of the mixed-lineage kinase DLK/MUK/ZPK to the Golgi apparatus in NIH 3T3 cells. J Histochem Cytochem 1999; 47: 1287–1296.
Zhang J, Lesort M, Guttmann RP, Johnson GVW . Modulation of the in situ activity of tissue transglutaminase by calcium and GTP. J Biol Chem 1998; 273: 2288–2295.
Acknowledgements
We thank Dr. Alain Lavigueur for critical reading of the article and Dr. Nicolas Gévry for his help with the design of the short hairpin sequences. This work was supported by a grant from the Canadian Institutes of Health Research (CIHR). KR was recipient of a studentship from the Fonds Québécois de la Recherche sur la Nature et les Technologies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by M Piacentini
Rights and permissions
About this article
Cite this article
Robitaille, K., Daviau, A., Lachance, G. et al. Calphostin C-induced apoptosis is mediated by a tissue transglutaminase-dependent mechanism involving the DLK/JNK signaling pathway. Cell Death Differ 15, 1522–1531 (2008). https://doi.org/10.1038/cdd.2008.77
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2008.77
Keywords
This article is cited by
-
Mechanistic role of transglutaminase-2 in focal adhesions
Scientific Reports (2018)
-
Transglutaminase 2 is involved in amyloid-beta1–42-induced pro-inflammatory activation via AP1/JNK signalling pathways in THP-1 monocytes
Amino Acids (2017)
-
Transglutaminase 2: a multi-functional protein in multiple subcellular compartments
Amino Acids (2010)
-
DLK
AfCS-Nature Molecule Pages (2009)
-
JNK signaling in apoptosis
Oncogene (2008)