Abstract
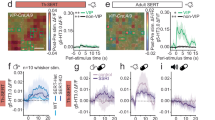
There has been a growing controversy regarding the continued use of glucocorticoid therapy to treat respiratory dysfunction associated with prematurity, as mounting clinical evidence has shown neonatal exposure produces permanent neuromotor and cognitive deficits. Here we report that, during a selective neonatal window of vulnerability, a single glucocorticoid injection in the mouse produces rapid and selective apoptotic cell death of the proliferating neural progenitor cells in the cerebellar external granule layer and permanent reductions in neuronal cell counts of their progeny, the cerebellar internal granule layer neurons. Our estimates suggest that this mouse window of vulnerability would correspond in the human to a period extending from approximately 20 weeks gestation to 6.5 weeks after birth. This death pathway is critically regulated by the proapoptotic Bcl-2 family member Puma and is independent of p53 expression. These rodent data indicate that there exists a previously unknown window of vulnerability during which a single glucocorticoid exposure at clinically relevant doses can produce neural progenitor cell apoptosis and permanent cerebellar pathology that may be responsible for some of the iatrogenically induced neurodevelopmental abnormalities seen in children exposed to this drug. This vulnerability may be related to the physiological role of glucocorticoids in regulating programmed cell death in the mammalian cerebellum.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ANOVA:
-
analysis of variance
- AraC:
-
cytosine arabinoside
- C3A:
-
activated caspase-3
- DEX:
-
dexamethasone
- EGL:
-
external granule layer
- GC:
-
glucocorticoid
- KO:
-
knockout
- NPC:
-
neural progenitor cell
- PND:
-
postnatal day
- WT:
-
wild type
References
Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens State 1994; 12: 1–24.
Antenatal corticosteroids revisited: repeat courses. NIH Consens State 2000; 17: 1–18.
Leviton LC, Goldenberg RL, Baker CS, Schwartz RM, Freda MC, Fish LJ et al. Methods to encourage the use of antenatal corticosteroid therapy for fetal maturation: a randomized controlled trial. JAMA 1999; 281: 46–52.
Halliday HL . The effect of postnatal steroids on growth and development. J Perinat Med 2001; 29: 281–285.
Grier DG, Halliday HL . Corticosteroids in the prevention and management of bronchopulmonary dysplasia. Semin Neonatol 2003; 8: 83–91.
Halliday HL . Use of steroids in the perinatal period. Paediatr Respir Rev 2004; 5 (Suppl A): S321–S327.
Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics 2002; 109: 330–338.
Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med 2004; 350: 1304–1313.
St John EB, Carlo WA . Respiratory distress syndrome in VLBW infants: changes in management and outcomes observed by the NICHD Neonatal Research Network. Semin Perinatol 2003; 27: 288–292.
Jobe AH, Soll RF . Choice and dose of corticosteroid for antenatal treatments. Am J Obstet Gynecol 2004; 190: 878–881.
Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 2000; 287: 1056–1060.
Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999; 283: 70–74.
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 2003; 23: 876–882.
Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D’Sa C et al. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis 2002; 9: 205–219.
De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M . Brain corticosteroid receptor balance in health and disease. Endocr Rev 1998; 19: 269–301.
Pavlik A, Buresova M . The neonatal cerebellum: the highest level of glucocorticoid receptors in the brain. Brain Res 1984; 314: 13–20.
Goldowitz D, Hamre K . The cells and molecules that make a cerebellum. Trends Neurosci 1998; 21: 375–382.
Shacka JJ, Roth KA . Bcl-2 family and the central nervous system: from rheostat to real complex. Cell Death Differ 2006; 13: 1299–1304.
Akhtar RS, Ness JM, Roth KA . Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta 2004; 1644: 189–203.
Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003; 302: 1036–1038.
Geng Y, Akhtar RS, Shacka JJ, Klocke BJ, Zhang J, Chen X et al. p53 transcription-dependent and -independent regulation of cerebellar neural precursor cell apoptosis. J Neuropathol Exp Neurol 2007; 66: 66–74.
Andersen BB, Korbo L, Pakkenberg B . A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol 1992; 326: 549–560.
de la Rosa EJ, de Pablo F . Cell death in early neural development: beyond the neurotrophic theory. Trends Neurosci 2000; 23: 454–458.
De Zio D, Giunta L, Corvaro M, Ferraro E, Cecconi F . Expanding roles of programmed cell death in mammalian neurodevelopment. Semin Cell Dev Biol 2005; 16: 281–294.
Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski JA, Jones KR et al. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature 2005; 435: 1244–1250.
Chizhikov V, Millen KJ . Development and malformations of the cerebellum in mice. Mol Genet Metab 2003; 80: 54–65.
Benesova O, Pavlik A . Perinatal treatment with glucocorticoids and the risk of maldevelopment of the brain. Neuropharmacology 1989; 28: 89–97.
Schmahmann JD . Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 2004; 16: 367–378.
Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B . Annual summary of vital statistics: 2004. Pediatrics 2006; 117: 168–183.
Schmidt MV, Oitzl MS, Levine S, de Kloet ER . The HPA system during the postnatal development of CD1 mice and the effects of maternal deprivation. Brain Res 2002; 139: 39–49.
Robson AC, Leckie CM, Seckl JR, Holmes MC . 11 beta-hydroxysteroid dehydrogenase type 2 in the postnatal and adult rat brain. Brain Res 1998; 61: 1–10.
Holmes MC, Sangra M, French KL, Whittle IR, Paterson J, Mullins JJ et al. 11 beta-Hydroxysteroid dehydrogenase type 2 protects the neonatal cerebellum from deleterious effects of glucocorticoids. Neuroscience 2006; 137: 865–873.
Medh RD, Thompson EB . Hormonal regulation of physiological cell turnover and apoptosis. Cell Tissue Res 2000; 301: 101–124.
Liggins GC . The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev 1994; 6: 141–150.
Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med 2006; 203: 2939–2951.
Sapolsky RM . Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol 1999; 34: 721–732.
Akhtar RS, Geng Y, Klocke BJ, Roth KA . Neural precursor cells possess multiple p53-dependent apoptotic pathways. Cell Death Differ 2006; 13: 1727–1739.
Rakic P, Sidman RL . Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J Comp Neurol 1970; 139: 473–500.
Fujita S, Shimada M, Nakamura T . H3-thymidine autoradiographic studies on the cell proliferation and differentiation in the external and the internal granular layers of the mouse cerebellum. J Comp Neurol 1966; 128: 191–208.
Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T et al. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis 2004; 17: 403–414.
Acknowledgements
KCW and KAR thank UAB Neuroscience Core Facilities (NS47466 and NS57098) for technical support. KAR and KCW are supported by NIH grants NS35107 and NS41962. Puma −/− mice were generously provided to us by Dr. Andreas Strasser (University of Melbourne). KKN, NBF, and JWO thank D Smith and H Wang for technical assistance. KKN is supported by NIH grants DA07261, DA05072, and HD055365. DFW is supported by an NIH P30 Neuroscience Blueprint Core Grant NS057105. NBF is supported by NIH grant ES12443 and HD055365. JWO is supported by NIH grant DA05072 and HD055365. Finally, we thank K Dikranian and A Parsadanian for advice and technical support with double immunofluorescent histology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by JA Cidlowski
Rights and permissions
About this article
Cite this article
Noguchi, K., Walls, K., Wozniak, D. et al. Acute neonatal glucocorticoid exposure produces selective and rapid cerebellar neural progenitor cell apoptotic death. Cell Death Differ 15, 1582–1592 (2008). https://doi.org/10.1038/cdd.2008.97
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2008.97
Keywords
This article is cited by
-
Single neonatal dexamethasone administration has long-lasting outcome on depressive-like behaviour, Bdnf, Nt-3, p75ngfr and sorting receptors (SorCS1-3) stress reactive expression
Scientific Reports (2021)
-
Sonic Hedgehog Agonist Protects Against Complex Neonatal Cerebellar Injury
The Cerebellum (2018)
-
Antidepressant responsiveness in adulthood is permanently impaired after neonatal destruction of the neurogenic pool
Translational Psychiatry (2017)
-
Maternal cortisol stimulates neurogenesis and affects larval behaviour in zebrafish
Scientific Reports (2017)
-
Nuclear receptors in neural stem/progenitor cell homeostasis
Cellular and Molecular Life Sciences (2017)