Abstract
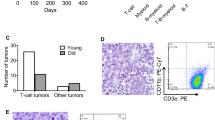
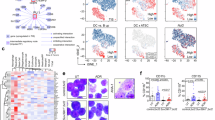
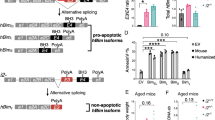
We propose that the apoptotic function of p53 has an important role in B-cell homeostasis, which is important for the prevention of B-cell lymphomas. We created a mouse model (mΔpro) that lacked residues 58–88 of the proline-rich domain of p53. mΔpro is defective for apoptosis, but is able to arrest cell-cycle progression in hematopoietic tissues. mΔpro develops late-onset B-cell lymphoma, but not the thymic T-cell tumors found in p53-null mice. Interestingly, mΔpro lymphomas comprised incorrectly differentiated B cells. B-cell irregularities were also detected in mΔpro before tumor onset, in which aged mice showed an increased population of inappropriately differentiated B cells in the bone marrow and spleen. We predict that by keeping B-cell populations in check, p53-dependent apoptosis prevents irregular B cells from eventuating in lymphomas.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- BrdU:
-
bromo-deoxyuridine
- ES:
-
embryonic stem cells
- Δ, deletion, Pin1:
-
prolyl isomerise 1
- PCR:
-
polymerase chain reaction
- TP53 :
-
human p53 gene
- Trp53 :
-
mouse p53 gene
References
Preudhomme C, Fenaux P . p53 and hematologic malignancies. Pathol Biol (Paris) 1997; 45: 898–908.
O'Shea D, O'Riain C, Taylor C, Waters R, Carlotti E, Macdougall F et al. The presence of TP53 mutation at diagnosis of follicular lymphoma identifies a high-risk group of patients with shortened time to disease progression and poorer overall survival. Blood 2008; 112: 3126–3129.
Young KH, Weisenburger DD, Dave BJ, Smith L, Sanger W, Iqbal J et al. Mutations in the DNA-binding codons of TP53, which are associated with decreased expression of TRAILreceptor-2, predict for poor survival in diffuse large B-cell lymphoma. Blood 2007; 110: 4396–4405.
Ichikawa A, Hotta T, Takagi N, Tsushita K, Kinoshita T, Nagai H et al. Mutations of p53 gene and their relation to disease progression in B-cell lymphoma. Blood 1992; 79: 2701–2707.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery Jr CA, Butel JS et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992; 356: 215–221.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994; 4: 1–7.
Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 2004; 119: 861–872.
Liu G, Parant JM, Lang G, Chau P, Chavez-Reyes A, El-Naggar AK et al. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet 2004; 36: 63–68.
MacPherson D, Kim J, Kim T, Rhee BK, Van Oostrom CT, DiTullio RA et al. Defective apoptosis and B-cell lymphomas in mice with p53 point mutation at Ser 23. EMBO J 2004; 23: 3689–3699.
Vousden KH, Lu X . Live or let die: the cell's response to p53. Nat Rev Cancer 2002; 2: 594–604.
Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L et al. Restoration of p53 function leads to tumour regression in vivo. Nature 2007; 445: 661–665.
Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007; 445: 656–660.
Walker KK, Levine AJ . Identification of a novel p53 functional domain that is necessary for efficient growth suppression. Proc Natl Acad Sci USA 1996; 93: 15335–15340.
Baptiste N, Friedlander P, Chen X, Prives C . The proline-rich domain of p53 is required for cooperation with anti-neoplastic agents to promote apoptosis of tumor cells. Oncogene 2002; 21: 9–21.
Zhu J, Jiang J, Zhou W, Zhu K, Chen X . Differential regulation of cellular target genes by p53 devoid of the PXXP motifs with impaired apoptotic activity. Oncogene 1999; 18: 2149–2155.
Edwards SJ, Hananeia L, Eccles MR, Zhang YF, Braithwaite AW . The proline-rich region of mouse p53 influences transactivation and apoptosis but is largely dispensable for these functions. Oncogene 2003; 22: 4517–4523.
Ruaro EM, Collavin L, Del Sal G, Haffner R, Oren M, Levine AJ et al. A proline-rich motif in p53 is required for transactivation-independent growth arrest as induced by Gas1. Proc Natl Acad Sci USA 1997; 94: 4675–4680.
Toledo F, Krummel KA, Lee CJ, Liu CW, Rodewald LW, Tang M et al. A mouse p53 mutant lacking the proline-rich domain rescues Mdm4 deficiency and provides insight into the Mdm2-Mdm4-p53 regulatory network. Cancer Cell 2006; 9: 273–285.
Toledo F, Lee CJ, Krummel KA, Rodewald LW, Liu CW, Wahl GM . Mouse mutants reveal that putative protein interaction sites in the p53 proline-rich domain are dispensable for tumor suppression. Mol Cell Biol 2007; 27: 1425–1432.
Johnson RK, Wodinsky I, Swiniarski J, Meaney KF, Clement JJ . Interaction of gamma-irradiation with two new antineoplastic agents, aziridinylbenzoquinone (AZQ) and 4′- (acridinylamino)methanesulfon-m-anisidide (AMSA), in murine tumors in vivo. Int J Radiat Oncol Biol Phys 1979; 5: 1605–1609.
Griffin RJ, Williams BW, Bischof JC, Olin M, Johnson GL, Lee BW . Use of a fluorescently labeled poly-caspase inhibitor for in vivo detection of apoptosis related to vascular-targeting agent arsenic trioxide for cancer therapy. Technol Cancer Res Treat 2007; 6: 651–654.
Samuels-Lev Y, O'Connor DJ, Bergamaschi D, Trigiante G, Hsieh JK, Zhong S et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell 2001; 8: 781–794.
Bergamaschi D, Samuels Y, Sullivan A, Zvelebil M, Breyssens H, Bisso A et al. iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72-polymorphic p53. Nat Genet 2006; 38: 1133–1141.
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004; 303: 1010–1014.
Chipuk JE, Maurer U, Green DR, Schuler M . Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer Cell 2003; 4: 371–381.
Attardi LD, Lowe SW, Brugarolas J, Jacks T . Transcriptional activation by p53, but not induction of the p21 gene, is essential for oncogene-mediated apoptosis. EMBO J 1996; 15: 3693–3701.
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T . p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 1993; 362: 847–849.
Roth J, Koch P, Contente A, Dobbelstein M . Tumor-derived mutations within the DNA-binding domain of p53 that phenotypically resemble the deletion of the proline-rich domain. Oncogene 2000; 19: 1834–1842.
Sakamuro D, Sabbatini P, White E, Prendergast GC . The polyproline region of p53 is required to activate apoptosis but not growth arrest. Oncogene 1997; 15: 887–898.
Venot C, Maratrat M, Dureuil C, Conseiller E, Bracco L, Debussche L . The requirement for the p53 proline-rich functional domain for mediation of apoptosis is correlated with specific PIG3 gene transactivation and with transcriptional repression. EMBO J 1998; 17: 4668–4679.
Hardy RR, Kincade PW, Dorshkind K . The protean nature of cells in the B lymphocyte lineage. Immunity 2007; 26: 703–714.
Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A . A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA 1992; 89: 6550–6554.
Liu YJ, Banchereau J . Mutant mice without B lymphocyte follicles. J Exp Med 1996; 184: 1207–1211.
Jimenez GS, Nister M, Stommel JM, Beeche M, Barcarse EA, Zhang XQ et al. A transactivation-deficient mouse model provides insights into Trp53 regulation and function. Nat Genet 2000; 26: 37–43.
Chao C, Saito S, Kang J, Anderson CW, Appella E, Xu Y . p53 transcriptional activity is essential for p53-dependent apoptosis following DNA damage. EMBO J 2000; 19: 4967–4975.
Sabbatini P, Lin J, Levine AJ, White E . Essential role for p53-mediated transcription in E1A-induced apoptosis. Genes Dev 1995; 9: 2184–2192.
Braun FK, Fecker LF, Schwarz C, Walden P, Assaf C, Durkop H et al. Blockade of death receptor-mediated pathways early in the signaling cascade coincides with distinct apoptosis resistance in cutaneous T-cell lymphoma cells. J Invest Dermatol 2007; 127: 2425–2437.
Klemke CD, Brenner D, Weiss EM, Schmidt M, Leverkus M, Gulow K et al. Lack of T-cell receptor-induced signaling is crucial for CD95 ligand up-regulation and protects cutaneous T-cell lymphoma cells from activation-induced cell death. Cancer Res 2009; 69: 4175–4183.
Chen J, Fiskus W, Eaton K, Fernandez P, Wang Y, Rao R et al. Cotreatment with BCL-2 antagonist sensitizes cutaneous T-cell lymphoma to lethal action of HDAC7-Nur77-based mechanism. Blood 2009; 113: 4038–4048.
Kampa KM, Acoba JD, Chen D, Gay J, Lee H, Beemer K et al. Apoptosis-stimulating protein of p53 (ASPP2) heterozygous mice are tumor-prone and have attenuated cellular damage-response thresholds. Proc Natl Acad Sci USA 2009; 106: 4390–4395.
Deng C, Zhang P, Harper JW, Elledge SJ, Leder P . Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995; 82: 675–684.
Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med 2006; 203: 2939–2951.
Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci USA 2003; 100: 9324–9329.
Feldser DM, Greider CW . Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell 2007; 11: 461–469.
Diamant E, Keren Z, Melamed D . CD19 regulates positive selection and maturation in B lymphopoiesis: lack of CD19 imposes developmental arrest of immature B cells and consequential stimulation of receptor editing. Blood 2005; 105: 3247–3254.
Rickert RC, Rajewsky K, Roes J . Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature 1995; 376: 352–355.
Engel P, Zhou LJ, Ord DC, Sato S, Koller B, Tedder TF . Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity 1995; 3: 39–50.
Koduru PR, Raju K, Vadmal V, Menezes G, Shah S, Susin M et al. Correlation between mutation in P53, p53 expression, cytogenetics, histologic type, and survival in patients with B-cell non-Hodgkin's lymphoma. Blood 1997; 90: 4078–4091.
Malkin D, Li FP, Strong LC, Fraumeni Jr JF, Nelson CE, Kim DH et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 1990; 250: 1233–1238.
Li FP, Fraumeni Jr JF . Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med 1969; 71: 747–752.
Li FP, Fraumeni Jr JF, Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA et al. A cancer family syndrome in twenty-four kindreds. Cancer Res 1988; 48: 5358–5362.
Nichols KE, Malkin D, Garber JE, Fraumeni Jr JF, Li FP . Germ-line p53 mutations predispose to a wide spectrum of early-onset cancers. Cancer Epidemiol Biomarkers Prev 2001; 10: 83–87.
Kimura M, Yamaguchi M, Nakamura S, Imai H, Ueno S, Ogawa S et al. Clinicopathologic significance of loss of CD19 expression in diffuse large B-cell lymphoma. Int J Hematol 2007; 85: 41–48.
Masir N, Marafioti T, Jones M, Natkunam Y, Rudiger T, Hansmann ML et al. Loss of CD19 expression in B-cell neoplasms. Histopathology 2006; 48: 239–246.
Tedoldi S, Mottok A, Ying J, Paterson JC, Cui Y, Facchetti F et al. Selective loss of B-cell phenotype in lymphocyte predominant Hodgkin lymphoma. J Pathol 2007; 213: 429–440.
Yang W, Agrawal N, Patel J, Edinger A, Osei E, Thut D et al. Diminished expression of CD19 in B-cell lymphomas. Cytometry B Clin Cytom 2005; 63: 28–35.
Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008; 132: 875–886.
Acknowledgements
We are grateful for technical assistance from N Bennett, J North, M Schultz, Z Lateef, B Li, X Tan, and L Wallis. L Hananeia and S Edwards are acknowledged for their contributions to the early phases of this study. I Morison, D Speidel, and H Campbell are thanked for comments on the paper. This work was supported by a grant from the Royal Society of New Zealand Marsden Fund, a Cancer Institute NSW Program Grant, and a Health Research Council of New Zealand project grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by M Oren
Rights and permissions
About this article
Cite this article
Slatter, T., Ganesan, P., Holzhauer, C. et al. p53-mediated apoptosis prevents the accumulation of progenitor B cells and B-cell tumors. Cell Death Differ 17, 540–550 (2010). https://doi.org/10.1038/cdd.2009.136
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2009.136
Keywords
This article is cited by
-
Silencing of Testin expression is a frequent event in spontaneous lymphomas from Trp53-mutant mice
Scientific Reports (2020)
-
∆133p53 isoform promotes tumour invasion and metastasis via interleukin-6 activation of JAK-STAT and RhoA-ROCK signalling
Nature Communications (2018)
-
Tumor protein 53 mutations are enriched in diffuse large B-cell lymphoma with irregular CD19 marker expression
Scientific Reports (2017)
-
The proline rich domain of p53 is dispensable for MGMT-dependent DNA repair and cell survival following alkylation damage
Cell Death & Differentiation (2017)
-
Δ122p53, a mouse model of Δ133p53α, enhances the tumor-suppressor activities of an attenuated p53 mutant
Cell Death & Disease (2015)