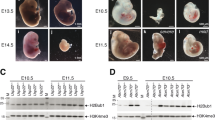
Abstract
Ubiquitin-mediated protein degradation is the main mechanism for controlled proteolysis, which is crucial for muscle development and maintenance. The ankyrin repeat-containing protein with a suppressor of cytokine signaling box 2 gene (ASB2) encodes the specificity subunit of an E3 ubiquitin ligase complex involved in differentiation of hematopoietic cells. Here, we provide the first evidence that a novel ASB2 isoform, ASB2β, is important for muscle differentiation. ASB2β is expressed in muscle cells during embryogenesis and in adult tissues. ASB2β is part of an active E3 ubiquitin ligase complex and targets the actin-binding protein filamin B (FLNb) for proteasomal degradation. Thus, ASB2β regulates FLNb functions by controlling its degradation. Knockdown of endogenous ASB2β by shRNAs during induced differentiation of C2C12 cells delayed FLNb degradation as well as myoblast fusion and expression of muscle contractile proteins. Finally, knockdown of FLNb in ASB2β knockdown cells restores myogenic differentiation. Altogether, our results suggest that ASB2β is involved in muscle differentiation through the targeting of FLNb to destruction by the proteasome.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
Abbreviations
- ASB2 :
-
ankyrin repeat-containing protein with a suppressor of cytokine signaling box 2 gene
- CRL:
-
Cullin RING ligase
- FLN:
-
filamin
- HECT:
-
homologous to the E6-associated protein C terminus
- MHC:
-
myosin heavy chain
- MURF:
-
muscle RING finger
- RING:
-
really interesting new gene
- SOCS:
-
suppressor of cytokine signaling
- UIM:
-
ubiquitin-interacting motif
- UPS:
-
ubiquitin–proteasome system
References
Glickman MH, Ciechanover A . The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 2002; 82: 373–428.
Hilton DJ, Richardson RT, Alexander WS, Viney EM, Willson TA, Sprigg NS et al. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA 1998; 95: 114–119.
Guibal FC, Moog-Lutz C, Smolewski P, Di Gioia Y, Darzynkiewicz Z, Lutz PG et al. ASB-2 inhibits growth and promotes commitment in myeloid leukemia cells. J Biol Chem 2002; 277: 218–224.
Moog-Lutz C, Cave-Riant F, Guibal FC, Breau MA, Di Gioia Y, Couraud PO et al. JAML, a novel protein with characteristics of a junctional adhesion molecule, is induced during differentiation of myeloid leukemia cells. Blood 2003; 102: 3371–3378.
Heuze ML, Guibal FC, Banks CA, Conaway JW, Conaway RC, Cayre YE et al. ASB2 is an Elongin BC-interacting protein that can assemble with Cullin 5 and Rbx1 to reconstitute an E3 ubiquitin ligase complex. J Biol Chem 2005; 280: 5468–5474.
Heuze ML, Lamsoul I, Baldassarre M, Lad Y, Lévêque S, Razinia Z et al. ASB2 targets filamins A and B to proteasomal degradation. Blood 2008; 112: 5130–5140.
Lindon C, Montarras D, Pinset C . Cell cycle-regulated expression of the muscle determination factor Myf5 in proliferating myoblasts. J Cell Biol 1998; 140: 111–118.
Song A, Wang Q, Goebl MG, Harrington MA . Phosphorylation of nuclear MyoD is required for its rapid degradation. Mol Cell Biol 1998; 18: 4994–4999.
Sun L, Trausch-Azar JS, Ciechanover A, Schwartz AL . Ubiquitin-proteasome-mediated degradation, intracellular localization, and protein synthesis of MyoD and Id1 during muscle differentiation. J Biol Chem 2005; 280: 26448–26456.
Sun L, Trausch-Azar JS, Ciechanover A, Schwartz AL . E2A protein degradation by the ubiquitin-proteasome system is stage-dependent during muscle differentiation. Oncogene 2007; 26: 441–448.
Solomon V, Goldberg AL . Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 1996; 271: 26690–26697.
Kudryashova E, Kudryashov D, Kramerova I, Spencer MJ . Trim32 is a ubiquitin ligase mutated in limb girdle muscular dystrophy type 2H that binds to skeletal muscle myosin and ubiquitinates actin. J Mol Biol 2005; 354: 413–424.
Witt SH, Granzier H, Witt CC, Labeit S . MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol 2005; 350: 713–722.
Zhao TJ, Yan YB, Liu Y, Zhou HM . The generation of the oxidized form of creatine kinase is a negative regulation on muscle creatine kinase. J Biol Chem 2007; 282: 12022–12029.
Pizon V, Iakovenko A, Van Der Ven PF, Kelly R, Fatu C, Furst DO et al. Transient association of titin and myosin with microtubules in nascent myofibrils directed by the MURF2 RING-finger protein. J Cell Sci 2002; 115: 4469–4482.
McElhinny AS, Perry CN, Witt CC, Labeit S, Gregorio CC . Muscle-specific RING finger-2 (MURF-2) is important for microtubule, intermediate filament and sarcomeric M-line maintenance in striated muscle development. J Cell Sci 2004; 117: 3175–3188.
Spencer JA, Eliazer S, Ilaria Jr RL, Richardson JA, Olson EN . Regulation of microtubule dynamics and myogenic differentiation by MURF, a striated muscle RING-finger protein. J Cell Biol 2000; 150: 771–784.
Fielitz J, van Rooij E, Spencer JA, Shelton JM, Latif S, van der Nagel R et al. Loss of muscle-specific RING-finger 3 predisposes the heart to cardiac rupture after myocardial infarction. Proc Natl Acad Sci USA 2007; 104: 4377–4382.
Fielitz J, Kim MS, Shelton JM, Latif S, Spencer JA, Glass DJ et al. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J Clin Invest 2007; 117: 2486–2495.
Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA . Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem 2005; 280: 2847–2856.
Nastasi T, Bongiovanni A, Campos Y, Mann L, Toy JN, Bostrom J et al. Ozz-E3, a muscle-specific ubiquitin ligase, regulates beta-catenin degradation during myogenesis. Dev Cell 2004; 6: 269–282.
McDaneld TG, Hannon K, Moody DE . Ankyrin repeat and SOCS box protein 15 regulates protein synthesis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2006; 290: R1672–R1682.
Li W, Wu G, Wan Y . The dual effects of Cdh1/APC in myogenesis. FASEB J 2007; 21: 3606–3617.
Kamura T, Maenaka K, Kotoshiba S, Matsumoto M, Kohda D, Conaway RC et al. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev 2004; 18: 3055–3065.
Young P, Deveraux Q, Beal RE, Pickart CM, Rechsteiner M . Characterization of two polyubiquitin binding sites in the 26 S protease subunit 5a. J Biol Chem 1998; 273: 5461–5467.
Hurley JH, Lee S, Prag G . Ubiquitin-binding domains. Biochem J 2006; 399: 361–372.
Flick K, Raasi S, Zhang H, Yen JL, Kaiser P . A ubiquitin-interacting motif protects polyubiquitinated Met4 from degradation by the 26S proteasome. Nat Cell Biol 2006; 8: 509–515.
van der Flier A, Kuikman I, Kramer D, Geerts D, Kreft M, Takafuta T et al. Different splice variants of filamin-B affect myogenesis, subcellular distribution, and determine binding to integrin [beta] subunits. J Cell Biol 2002; 156: 361–376.
Dedieu S, Poussard S, Mazeres G, Grise F, Dargelos E, Cottin P et al. Myoblast migration is regulated by calpain through its involvement in cell attachment and cytoskeletal organization. Exp Cell Res 2004; 292: 187–200.
Andres V, Walsh K . Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol 1996; 132: 657–666.
Menko AS, Boettiger D . Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell 1987; 51: 51–57.
Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J et al. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell 2003; 4: 673–685.
Calderwood DA, Huttenlocher A, Kiosses WB, Rose DM, Woodside DG, Schwartz MA et al. Increased filamin binding to beta-integrin cytoplasmic domains inhibits cell migration. Nat Cell Biol 2001; 3: 1060–1068.
McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH et al. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics 2007; 31: 86–95.
Edom F, Mouly V, Barbet JP, Fiszman MY, Butler-Browne GS . Clones of human satellite cells can express in vitro both fast and slow myosin heavy chains. Dev Biol 1994; 164: 219–229.
Lad Y, Kiema T, Jiang P, Pentikainen OT, Coles CH, Campbell ID et al. Structure of three tandem filamin domains reveals auto-inhibition of ligand binding. EMBO J 2007; 26: 3993–4004.
Chomczynski P, Sacchi N . Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162: 156–159.
Lutz PG, Houzel-Charavel A, Moog-Lutz C, Cayre YE . Myeloblastin is an Myb target gene: mechanisms of regulation in myeloid leukemia cells growth-arrested by retinoic acid. Blood 2001; 97: 2449–2456.
Tozer S, Bonnin MA, Relaix F, Di Savino S, Garcia-Villalba P, Coumailleau P et al. Involvement of vessels and PDGFB in muscle splitting during chick limb development. Development 2007; 134: 2579–2591.
Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, Wegener KL et al. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell 2006; 21: 337–347.
Acknowledgements
We thank J Dubrulle and O Pourquié for the chicken ASB2 probe and initial help with in situ hybridizations, M-A Bonnin for technical assistance with in situ hybridizations and V Mouly and the human cell culture platform from the Myology Institute in Paris for providing human primary myoblasts. We are grateful to D Heard for the design of shRNAs directed against mouse ASB2β. We thank Lucie Carrier for critical reading of the article. This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Université de Toulouse, the Université Pierre et Marie Curie and by grants to DAC from the National Institutes of Health (GM068600 and HL089433), to CML from the Université Paul Sabatier and to PGL from the Agence Nationale de la Recherche (Programme Jeunes Chercheuses, Jeunes Chercheurs), the Association Française contre les Myopathies, the Association pour la Recherche sur le Cancer (Programme Equipe Nouvelle) and the Fondation pour la Recherche Médicale (Programme Installation d'une nouvelle équipe). NF Bello is supported by the Association Française contre les Myopathies. ML Heuzé was supported by a doctoral Allocation de Recherche du Ministère de la Recherche et des Technologies and by the Association pour la Recherche sur le Cancer.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Edited by G Cossu
Rights and permissions
About this article
Cite this article
Bello, N., Lamsoul, I., Heuzé, M. et al. The E3 ubiquitin ligase specificity subunit ASB2β is a novel regulator of muscle differentiation that targets filamin B to proteasomal degradation. Cell Death Differ 16, 921–932 (2009). https://doi.org/10.1038/cdd.2009.27
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2009.27
Keywords
This article is cited by
-
Dynamics of skeletal muscle-resident stem cells during myogenesis in fibrodysplasia ossificans progressiva
npj Regenerative Medicine (2022)
-
Neuromuscular junction-specific genes screening by deep RNA-seq analysis
Cell & Bioscience (2021)
-
The dependency of autophagy and ubiquitin proteasome system during skeletal muscle atrophy
Biophysical Reviews (2021)
-
The notch pathway promotes NF-κB activation through Asb2 in T cell acute lymphoblastic leukemia cells
Cellular & Molecular Biology Letters (2018)
-
High-throughput analysis of the RNA-induced silencing complex in myotonic dystrophy type 1 patients identifies the dysregulation of miR-29c and its target ASB2
Cell Death & Disease (2018)