Abstract
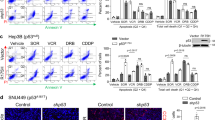
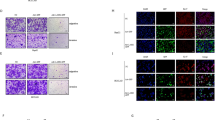
Stress protein mortalin is a multifunctional protein and is highly expressed in cancers. It has been shown to interact with tumor suppressor protein-p53 (both wild and mutant types) and inactivates its transcriptional activation and apoptotic functions in cancer cells. In the present study, we found that, unlike most of the cancer cells, HepG2 hepatoma lacked mortalin–p53 interaction. We demonstrate that the mortalin–p53 interaction exists in cancer cells that are either physiologically stressed (frequently associated with p53 mutations) or treated with stress-inducing chemicals. Targeting mortalin–p53 interaction with either mortalin small hairpin RNA or a chemical or peptide inhibitor could induce p53-mediated tumor cell-specific apoptosis in hepatocellular carcinoma; p53-null hepatoma or normal hepatocytes remain unaffected.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
Abbreviations
- shRNA:
-
small hairpin RNA
- Hsp70:
-
heat shock protein 70
- HCC:
-
hepatocellular carcinoma
- GRP78:
-
glucose-regulated protein 78
- MKT-077:
-
1-ethyl-2-[[3-ethyl-5-(3-methylbenzothiazolin-2-yliden)]-4-oxothiazolidin-2-ylidenemethyl]pyridinium chloride
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
References
Kastan MB, Zhan QM, Eldeiry WS, Carrier F, Jacks T, Walsh WV et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 1992; 71: 587–597.
Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M . Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 1991; 352: 345–347.
Nikolaev AY, Li M, Puskas N, Qin J, Gu W . Parc: a cytoplasmic anchor for p53. Cell 2003; 112: 29–40.
Zhao LY, Liao DQ . Sequestration of p53 in the cytoplasm by adenovirus type 12 E1B 55-kilodalton oncoprotein is required for inhibition of p53-mediated apoptosis. J Virol 2003; 77: 13171–13181.
Liu S, Li J, Tao Y, Xiao X . Small heat shock protein alphaB-crystallin binds to p53 to sequester its translocation to mitochondria during hydrogen peroxide-induced apoptosis. Biochem Biophys Res Commun 2007; 354: 109–114.
Murray-Zmijewski F, Slee EA, Lu X . A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol 2008; 9: 702–712.
Bode AM, Dong Z . Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 2004; 4: 793–805.
Luo J, Su F, Chen D, Shiloh A, Gu W . Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature 2000; 408: 377–381.
Melchior F, Hengst L . SUMO-1 and p53. Cell Cycle 2002; 1: 245–249.
Shaw P, Freeman J, Bovey R, Iggo R . Regulation of specific DNA binding by p53: evidence for a role for O-glycosylation and charged residues at the carboxy-terminus. Oncogene 1996; 12: 921–930.
Wesierska-Gadek J, Bugajska-Schretter A, Cerni C . ADP-ribosylation of p53 tumor suppressor protein: mutant but not wild-type p53 is modified. J Cell Biochem 1996; 62: 90–101.
Fuchs SY, Adler V, Buschmann T, Wu X, Ronai Z . Mdm2 association with p53 targets its ubiquitination. Oncogene 1998; 17: 2543–2547.
Sugito K, Yamane M, Hattori H, Hayashi Y, Tohnai I, Ueda M et al. Interaction between hsp70 and hsp40, eukaryotic homologues of DnaK and DnaJ, in human cells expressing mutant-type p53. FEBS Lett 1995; 358: 161–164.
Selkirk JK, Merrick BA, Stackhouse BL, He C . Multiple p53 protein isoforms and formation of oligomeric complexes with heat shock proteins Hsp70 and Hsp90 in the human mammary tumor, T47D, cell line. Appl Theor Electrophor 1994; 4: 11–18.
Sepehrnia B, Paz IB, Dasgupta G, Momand J . Heat shock protein 84 forms a complex with mutant p53 protein predominantly within a cytoplasmic compartment of the cell. J Biol Chem 1996; 271: 15084–15090.
Dundas SR, Lawrie LC, Rooney PH, Murray GI . Mortalin is over-expressed by colorectal adenocarcinomas and correlates with poor survival. J Pathol 2005; 205: 74–81.
Wadhwa R, Takano S, Kaur K, Deocaris CC, Pereira-Smith OM, Reddel RR et al. Upregulation of mortalin/mthsp70/Grp75 contributes to human carcinogenesis. Int J Cancer 2006; 118: 2973–2980.
Yi X, Luk JM, Lee NP, Peng J, Leng X, Guan XY et al. Association of mortalin (HSPA9) with liver cancer metastasis and prediction for early tumor recurrence. Mol Cell Proteomics 2008; 7: 315–325.
Kaul SC, Aida S, Yaguchi T, Kaur K, Wadhwa R . Activation of wild type p53 function by its mortalin-binding, cytoplasmically localizing carboxyl terminus peptides. J Biol Chem 2005; 280: 39373–39379.
Wadhwa R, Takano S, Robert M, Yoshida A, Nomura H, Reddel RR et al. Inactivation of tumor suppressor p53 by mot-2, a hsp70 family member. J Biol Chem 1998; 273: 29586–29591.
Ma Z, Izumi H, Kanai M, Kabuyama Y, Ahn NG, Fukasawa K . Mortalin controls centrosome duplication via modulating centrosomal localization of p53. Oncogene 2006; 25: 5377–5390.
Kanai M, Ma Z, Izumi H, Kim SH, Mattison CP, Winey M et al. Physical and functional interaction between mortalin and Mps1 kinase. Genes Cells 2007; 12: 797–810.
Wadhwa R, Sugihara T, Yoshida A, Nomura H, Reddel RR, Simpson R et al. Selective toxicity of MKT-077 to cancer cells is mediated by its binding to the hsp70 family protein mot-2 and reactivation of p53 function. Cancer Res 2000; 60: 6818–6821.
Ran Q, Wadhwa R, Kawai R, Kaul SC, Sifers RN, Bick RJ et al. Extra-mitochondrial localization of mortalin/mthsp70/PBP74/GRP75. Biochem Biophys Res Commun 2000; 275: 174–179.
Wadhwa R, Taira K, Kaul S C 2002 An hsp70 family chaperone, mortalin: what, when and where? Cell Stress Chaperones 7: 309–316.
Strom E, Sathe S, Komarov PG, Chernova OB, Pavlovska I, Shyshynova I et al. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat Chem Biol 2006; 2: 474–479.
Leu JI, Pimkina J, Frank A, Murphy ME, George DL . A small molecule inhibitor of inducible heat shock protein 70. Mol Cell 2009; 36: 15–27.
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004; 303: 1010–1014.
Lowe SW, Cepero E, Evan G . Intrinsic tumour suppression. Nature 2004; 432: 307–315.
Dearth LR, Qian H, Wang T, Baroni TE, Zeng J, Chen SW et al. Inactive full-length p53 mutants lacking dominant wild-type p53 inhibition highlight loss of heterozygosity as an important aspect of p53 status in human cancers. Carcinogenesis 2007; 28: 289–298.
Kato S, Han SY, Liu W, Otsuka K, Shibata H, Kanamaru R et al. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc Natl Acad Sci USA 2003; 100: 8424–8429.
Ishibashi J, Yamashit K, Ishikawa T, Hosokawa H, Sumida K, Nagayama M et al. The effects inhibiting the proliferation of cancer cells by far-infrared radiation (FIR) are controlled by the basal expression level of heat shock protein (HSP) 70A. Med Oncol 2008; 25: 229–237.
Hunziker A, Jensen MH, Krishna S . Stress-specific response of the p53-Mdm2 feedback loop. BMC Syst Biol 2010; 4: 94–98.
Luk JM, Lam CT, Siu AFM, Lam BY, Ng IOL, Hu MY et al. Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values. Proteomics 2006; 6: 1049–1057.
Nylandsted J, Wick W, Hirt UA, Brand K, Rohde M, Leist M et al. Eradication of glioblastoma, and breast and colon carcinoma xenografts by Hsp70 depletion. Cancer Res 2002; 62: 7139–7142.
Raffo AJ, Kim AL, Fine RL . Formation of nuclear Bax/p53 complexes is associated with chemotherapy induced apoptosis. Oncogene 2000; 19: 6216–6228.
Geng Y, Walls KC, Ghosh AP, Akhtar RS, Klocke BJ, Roth KA . Cytoplasmic p53 and activated Bax regulate p53-dependent, transcription-independent neural precursor cell apoptosis. J Histochem Cytochem 2010; 58: 265–275.
Brown JJ, Parashar B, Moshage H, Tanaka KE, Engelhardt D, Rabbani E et al. A long-term hepatitis B viremia model generated by transplanting nontumorigenic immortalized human hepatocytes in Rag-2-deficient mice. Hepatology 2000; 31: 173–181.
Wadhwa R, Takano S, Kaur K, Aida S, Yaguchi T, Kaul Z et al. Identification and characterization of molecular interactions between mortalin/mtHsp70 and HSP60. Biochem J 2005; 391: 185–190.
Liu LX, Lee NP, Chan VW, Xue W, Zender L et al. Targeting cadherin-17 inactivates Wnt signaling and inhibits tumor growth in liver carcinoma. Hepatology 2009; 50: 1453–1463.
Acknowledgements
This work was supported in part by the grants from the NUS seed fund, NMRC grant from the Ministry of Health, Singapore, and grant from the National Institute of Advanced Industrial Science & Technology (AIST).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by JC Marine
Rights and permissions
About this article
Cite this article
Lu, WJ., Lee, N., Kaul, S. et al. Mortalin–p53 interaction in cancer cells is stress dependent and constitutes a selective target for cancer therapy. Cell Death Differ 18, 1046–1056 (2011). https://doi.org/10.1038/cdd.2010.177
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2010.177
Keywords
This article is cited by
-
Pre-clinical safety and therapeutic efficacy of a plant-based alkaloid in a human colon cancer xenograft model
Cell Death Discovery (2022)
-
Onosma bracteata Wall. induces G0/G1 arrest and apoptosis in MG-63 human osteosarcoma cells via ROS generation and AKT/GSK3β/cyclin E pathway
Environmental Science and Pollution Research (2021)
-
Novel role of mortalin in attenuating HIV-1 Tat-mediated astrogliosis
Journal of Neuroinflammation (2020)
-
Mortalin/HSPA9 targeting selectively induces KRAS tumor cell death by perturbing mitochondrial membrane permeability
Oncogene (2020)
-
Wild type p53 function in p53Y220C mutant harboring cells by treatment with Ashwagandha derived anticancer withanolides: bioinformatics and experimental evidence
Journal of Experimental & Clinical Cancer Research (2019)