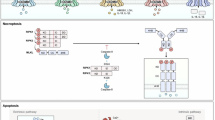
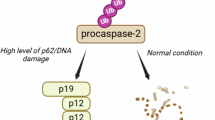
Abstract
Despite an abundance of literature on the role of caspase-2 in apoptosis, there exists much controversy about this protease making it difficult to place caspase-2 correctly in the apoptotic cascade, and hence its role in apoptosis remains unclear. The identification of the PIDDosome as a signaling platform for caspase-2 activation prompted intense investigation into the true role of this orphan caspase. What has emerged is the idea that caspase-2 may not be mandatory for apoptosis and that activation of this caspase in response to some forms of stress has other effects on the cell such as regulation of cell cycle progression. This idea is particularly relevent to the discovery that caspase-2 may act as a tumor suppressor. Here, we discuss the proposed mechanisms through which caspase-2 signals, in particular those involving PIDD, and their impact on cellular fate.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ALL:
-
acute lymphoblastic leukemia
- APAF-1:
-
apoptotic protease-activating factor-1
- ATM:
-
ataxia telangiectasia mutated
- CARD:
-
caspase recruitment domain
- Chk1:
-
checkpoint kinase 1
- DD:
-
death domain
- IκB:
-
inhibitor of κB
- LRR:
-
leucine-rich repeat
- Mdm2:
-
mammalian double minute 2
- NALP, NACHT:
-
LRR and PYD domains-containing protein
- NEMO:
-
NFκB essential modulator
- NFκB:
-
nuclear factor κB
- NOD:
-
nucleotide-binding oligomerization domain
- PCNA:
-
proliferating cell nuclear antigen
- PIDD:
-
p53-induced protein with a death domain
- PP1:
-
protein phosphatase 1
- RAIDD:
-
RIP-associated ICH-1/CAD-3 homologous protein with a death domain
- RIPK1:
-
receptor interacting protein kinase 1
- SUMO:
-
small ubiquitin-like modifier
- TNF:
-
tumor necrosis factor
- TNFR1:
-
tumor necrosis factor receptor 1
- TRADD:
-
tumor necrosis factor receptor type 1-associated death domain protein
References
Creagh EM, Conroy H, Martin SJ . Caspase-activation pathways in apoptosis and immunity. Immunol Rev 2003; 193: 10–21.
Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P . Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ 2002; 9: 358–361.
Krumschnabel G, Sohm B, Bock F, Manzl C, Villunger A . The enigma of caspase-2: the laymen′s view. Cell Death Differ 2009; 16: 195–207.
Vakifahmetoglu-Norberg H, Zhivotovsky B . The unpredictable caspase-2: what can it do? Trends Cell Biol 2010; 20: 150–159.
Boatright KM, Salvesen GS . Mechanisms of caspase activation. Curr Opin Cell Biol 2003; 15: 725–731.
Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM et al. A unified model for apical caspase activation. Mol Cell 2003; 11: 529–541.
Green DR . Apoptotic pathways: ten minutes to dead. Cell 2005; 121: 671–674.
Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D et al. Substrate specificities of caspase family proteases. J Biol Chem 1997; 272: 9677–9682.
Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 1997; 272: 17907–17911.
Baliga BC, Read SH, Kumar S . The biochemical mechanism of caspase-2 activation. Cell Death Differ 2004; 11: 1234–1241.
Read SH, Baliga BC, Ekert PG, Vaux DL, Kumar S . A novel Apaf-1-independent putative caspase-2 activation complex. J Cell Biol 2002; 159: 739–745.
Xue D, Shaham S, Horvitz HR . The Caenorhabditis elegans cell-death protein CED-3 is a cysteine protease with substrate specificities similar to those of the human CPP32 protease. Genes Dev 1996; 10: 1073–1083.
Hofmann K, Bucher P, Tschopp J . The CARD domain: a new apoptotic signalling motif. Trends Biochem Sci 1997; 22: 155–156.
Bouchier-Hayes L, Martin SJ . CARD games in apoptosis and immunity. EMBO Rep 2002; 3: 616–621.
Duan H, Dixit VM . RAIDD is a new ‘death’ adaptor molecule. Nature 1997; 385: 86–89.
Chou JJ, Matsuo H, Duan H, Wagner G . Solution structure of the RAIDD CARD and model for CARD/CARD interaction in caspase-2 and caspase-9 recruitment. Cell 1998; 94: 171–180.
Berube C, Boucher LM, Ma W, Wakeham A, Salmena L, Hakem R et al. Apoptosis caused by p53-induced protein with death domain (PIDD) depends on the death adapter protein RAIDD. Proc Natl Acad Sci USA 2005; 102: 14314–14320.
Tinel A, Tschopp J . The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 2004; 304: 843–846.
Lin Y, Ma W, Benchimol S . Pidd, a new death-domain-containing protein, is induced by p53 and promotes apoptosis. Nat Genet 2000; 26: 122–127.
Telliez JB, Bean KM, Lin LL . LRDD, a novel leucine rich repeat and death domain containing protein. Biochim Biophys Acta 2000; 1478: 280–288.
Aravind L, Dixit VM, Koonin EV . The domains of death: evolution of the apoptosis machinery. Trends Biochem Sci 1999; 24: 47–53.
Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB . The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature 1997; 386: 838–842.
Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M . Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 1997; 386: 833–838.
Kobe B, Deisenhofer J . The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci 1994; 19: 415–421.
Petrilli V, Dostert C, Muruve DA, Tschopp J . The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol 2007; 19: 615–622.
Inohara N, Nunez G . NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol 2003; 3: 371–382.
Tinel A, Janssens S, Lippens S, Cuenin S, Logette E, Jaccard B et al. Autoproteolysis of PIDD marks the bifurcation between pro-death caspase-2 and pro-survival NF-kappaB pathway. EMBO J 2007; 26: 197–208.
Hu Y, Ding L, Spencer DM, Nunez G . WD-40 repeat region regulates Apaf-1 self-association and procaspase-9 activation. J Biol Chem 1998; 273: 33489–33494.
Martinon F, Tschopp J . NLRs join TLRs as innate sensors of pathogens. Trends Immunol 2005; 26: 447–454.
Park HH, Logette E, Raunser S, Cuenin S, Walz T, Tschopp J et al. Death domain assembly mechanism revealed by crystal structure of the oligomeric PIDDosome core complex. Cell 2007; 128: 533–546.
Janssens S, Tinel A, Lippens S, Tschopp J . PIDD mediates NF-kappaB activation in response to DNA damage. Cell 2005; 123: 1079–1092.
Seeler JS, Dejean A . Nuclear and unclear functions of SUMO. Nat Rev Mol Cell Biol 2003; 4: 690–699.
Huang TT, Wuerzberger-Davis SM, Wu ZH, Miyamoto S . Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell 2003; 115: 565–576.
Wu ZH, Mabb A, Miyamoto S . PIDD: a switch hitter. Cell 2005; 123: 980–982.
Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES . Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J Biol Chem 2002; 277: 13430–13437.
Bonzon C, Bouchier-Hayes L, Pagliari LJ, Green DR, Newmeyer DD . Caspase-2-induced apoptosis requires bid cleavage: a physiological role for bid in heat shock-induced death. Mol Biol Cell 2006; 17: 2150–2157.
Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ . Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science 2000; 290: 1761–1765.
Mancini M, Machamer CE, Roy S, Nicholson DW, Thornberry NA, Casciola-Rosen LA et al. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J Cell Biol 2000; 149: 603–612.
Truscott M, Denault JB, Goulet B, Leduy L, Salvesen GS, Nepveu A . Carboxyl-terminal proteolytic processing of CUX1 by a caspase enables transcriptional activation in proliferating cells. J Biol Chem 2007; 282: 30216–30226.
Dahal GR, Karki P, Thapa A, Shahnawaz M, Shin SY, Lee JS et al. Caspase-2 cleaves DNA fragmentation factor (DFF45)/inhibitor of caspase-activated DNase (ICAD). Arch Biochem Biophys 2007; 468: 134–139.
Kitevska T, Spencer DM, Hawkins CJ . Caspase-2: controversial killer or checkpoint controller? Apoptosis 2009; 14: 829–848.
O′Reilly LA, Ekert P, Harvey N, Marsden V, Cullen L, Vaux DL et al. Caspase-2 is not required for thymocyte or neuronal apoptosis even though cleavage of caspase-2 is dependent on both Apaf-1 and caspase-9. Cell Death Differ 2002; 9: 832–841.
Bouchier-Hayes L, Oberst A, McStay GP, Connell S, Tait SW, Dillon CP et al. Characterization of cytoplasmic caspase-2 activation by induced proximity. Mol Cell 2009; 35: 830–840.
Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A et al. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev 1998; 12: 1304–1314.
Manzl C, Krumschnabel G, Bock F, Sohm B, Labi V, Baumgartner F et al. Caspase-2 activation in the absence of PIDDosome formation. J Cell Biol 2009; 185: 291–303.
Kim IR, Murakami K, Chen NJ, Saibil SD, Matysiak-Zablocki E, Elford AR et al. DNA damage- and stress-induced apoptosis occurs independently of PIDD. Apoptosis 2009; 14: 1039–1049.
Tu S, McStay GP, Boucher LM, Mak T, Beere HM, Green DR . In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nat Cell Biol 2006; 8: 72–77.
Shelton SN, Dillard CD, Robertson JD . Activation of caspase-9, but not caspase-2 or caspase-8, is essential for heat-induced apoptosis in Jurkat cells. J Biol Chem 2010; 285: 40525–40533.
Ho LH, Read SH, Dorstyn L, Lambrusco L, Kumar S . Caspase-2 is required for cell death induced by cytoskeletal disruption. Oncogene 2008; 27: 3393–3404.
Ho LH, Taylor R, Dorstyn L, Cakouros D, Bouillet P, Kumar S . A tumor suppressor function for caspase-2. Proc Natl Acad Sci USA 2009; 106: 5336–5341.
Kamer I, Sarig R, Zaltsman Y, Niv H, Oberkovitz G, Regev L et al. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell 2005; 122: 593–603.
Kaufmann T, Tai L, Ekert PG, Huang DC, Norris F, Lindemann RK et al. The BH3-only protein bid is dispensable for DNA damage- and replicative stress-induced apoptosis or cell-cycle arrest. Cell 2007; 129: 423–433.
Andersen JL, Johnson CE, Freel CD, Parrish AB, Day JL, Buchakjian MR et al. Restraint of apoptosis during mitosis through interdomain phosphorylation of caspase-2. Embo J 2009; 28: 3216–3227.
Oliver TG, Meylan E, Chang GP, Xue W, Burke JR, Humpton TJ et al. Caspase-2-mediated cleavage of Mdm2 creates a p53-induced positive feedback loop. Mol Cell 2011; 43: 57–71.
Tiwari M, Lopez-Cruzan M, Morgan WW, Herman B . Loss of caspase-2-dependent apoptosis induces autophagy after mitochondrial oxidative stress in primary cultures of young adult cortical neurons. J Biol Chem 2011; 286: 8493–8506.
Vousden KH, Ryan KM . p53 and metabolism. Nat Rev Cancer 2009; 9: 691–700.
Sidi S, Sanda T, Kennedy RD, Hagen AT, Jette CA, Hoffmans R et al. Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and caspase-3. Cell 2008; 133: 864–877.
Kumar S . Inhibition of apoptosis by the expression of antisense Nedd2. FEBS Lett 1995; 368: 69–72.
Mitelman F, Kaneko Y, Trent JM . Report of the committee on chromosome changes in neoplasia. Cytogenet Cell Genet 1990; 55: 358–386.
Hofmann WK, de Vos S, Tsukasaki K, Wachsman W, Pinkus GS, Said JW et al. Altered apoptosis pathways in mantle cell lymphoma detected by oligonucleotide microarray. Blood 2001; 98: 787–794.
Holleman A, den Boer ML, Kazemier KM, Beverloo HB, von Bergh AR, Janka-Schaub GE et al. Decreased PARP and procaspase-2 protein levels are associated with cellular drug resistance in childhood acute lymphoblastic leukemia. Blood 2005; 106: 1817–1823.
Estrov Z, Thall PF, Talpaz M, Estey EH, Kantarjian HM, Andreeff M et al. Caspase 2 and caspase 3 protein levels as predictors of survival in acute myelogenous leukemia. Blood 1998; 92: 3090–3097.
Faderl S, Thall PF, Kantarjian HM, Talpaz M, Harris D, Van Q et al. Caspase 2 and caspase 3 as predictors of complete remission and survival in adults with acute lymphoblastic leukemia. Clin Cancer Res 1999; 5: 4041–4047.
Kim MS, Chung NG, Yoo NJ, Lee SH . Somatic mutation of proapoptotic caspase-2 gene is rare in acute leukemias and common solid cancers. Eur J Haematol 2011; 86: 449–450.
Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 2003; 425: 407–410.
Bagatell R, Whitesell L . Altered Hsp90 function in cancer: a unique therapeutic opportunity. Mol Cancer Ther 2004; 3: 1021–1030.
Tinel A, Eckert MJ, Logette E, Lippens S, Janssens S, Jaccard B et al. Regulation of PIDD auto-proteolysis and activity by the molecular chaperone Hsp90. Cell Death Differ 2011; 18: 506–515.
Zhang Y, Padalecki SS, Chaudhuri AR, De Waal E, Goins BA, Grubbs B et al. Caspase-2 deficiency enhances aging-related traits in mice. Mech Ageing Dev 2007; 128: 213–221.
Coe LM, Lippner D, Perez GI, McCabe LR . Caspase 2 deficiency protects mice from diabetes-induced marrow adiposity. J Cell Biochem 2011; 112: 2403–2411.
Logette E, Schuepbach-Mallepell S, Eckert MJ, Leo XH, Jaccard B, Manzl C et al. PIDD orchestrates translesion DNA synthesis in response to UV irradiation. Cell Death Differ 2011; 18: 1036–1045.
Acknowledgements
It is with immeasurable sadness that we offer this small testimony to the life and a few of the myriad contributions of our friend, Professor Jürg Tschopp. Jürg was a valued colleague, great friend and a brilliant scientist. One other small contribution is noted in another field, that of skiing; Dr Tschopp was the first to force one of us (DRG) to navigate the treacherous double-black diamond run, called ‘The Devil′s Crotch’ on Breckenridge Mountain (Jürg skied it effortlessly, while DRG did it significantly less so, but the latter has skied it since with fond memories). We will miss you in so many ways, Jürg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Rights and permissions
About this article
Cite this article
Bouchier-Hayes, L., Green, D. Caspase-2: the orphan caspase. Cell Death Differ 19, 51–57 (2012). https://doi.org/10.1038/cdd.2011.157
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2011.157
Keywords
This article is cited by
-
BID expression determines the apoptotic fate of cancer cells after abrogation of the spindle assembly checkpoint by AURKB or TTK inhibitors
Molecular Cancer (2023)
-
The caspase-2 substrate p54nrb exhibits a multifaceted role in tumor cell death susceptibility via gene regulatory functions
Cell Death & Disease (2022)
-
Effects of intravitreal injection of siRNA against caspase-2 on retinal and optic nerve degeneration in air blast induced ocular trauma
Scientific Reports (2021)
-
Uncovering the PIDDosome and caspase-2 as regulators of organogenesis and cellular differentiation
Cell Death & Differentiation (2020)
-
Potent and selective caspase-2 inhibitor prevents MDM-2 cleavage in reversine-treated colon cancer cells
Cell Death & Differentiation (2019)