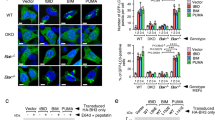
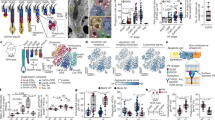
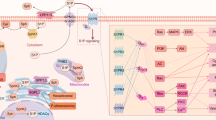
Abstract
Programmed cell death eliminates unwanted cells during normal development and physiological homeostasis. While cell interactions can influence apoptosis as they do other types of cell fate, outside of the adaptive immune system little is known about the intercellular cues that actively promote cell death in healthy cells. We used the Caenorhabditis elegans germline as a model to investigate the extrinsic regulators of physiological apoptosis. Using genetic and cell biological methods, we show that somatic gonad sheath cells, which also act as phagocytes of dying germ cells, promote death in the C. elegans germline through VAB-1/Eph receptor signaling. We report that the germline apoptosis function of VAB-1 impacts specific cell death pathways, and may act in parallel to extracellular signal-regulated kinase MAPK signaling. This work defines a non-autonomous, pro-apoptotic signaling for efficient physiological cell death, and highlights the dynamic nature of intercellular communication between dying cells and the phagocytes that remove them.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ERK:
-
extracellular signal-regulated kinase
- EphR:
-
Eph receptor
References
Kerr JF, Wyllie AH, Currie AR . Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–257.
Fadeel B, Orrenius S . Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med 2005; 258: 479–517.
Gurzov EN, Eizirik DL . Bcl-2 proteins in diabetes: mitochondrial pathways of Î2-cell death and dysfunction. Trends Cell Biol 2011; 21: 424–431.
Ruffin N, Ahmed SS, Osorio LM, Wang XN, Jackson GH, Collin MP et al. The involvement of epithelial Fas in a human model of graft versus host disease. Transplantation 2011; 91: 946–951.
Brumatti G, Salmanidis M, Ekert PG . Crossing paths: interactions between the cell death machinery and growth factor survival signals. Cell Mol Life Sci 2010; 67: 1619–1630.
Stupack DG, Cheresh DA . Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci 2002; 115 (Pt 19): 3729–3738.
Guicciardi ME, Gores GJ . Life and death by death receptors. FASEB J 2009; 23: 1625–1637.
O’Reilly LA, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature 2009; 461: 659–663.
Chavez-Galan L, Arenas-Del Angel MC, Zenteno E, Chavez R, Lascurain R . Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol 2009; 6: 15–25.
Gartner A, Boag PR, Blackwell TK . Germline survival and apoptosis. WormBook 2008 (http://www.wormbook.org).
Baum JS, St George JP, McCall K . Programmed cell death in the germline. Semin Cell Dev Biol 2005; 16: 245–259.
Wolke U, Jezuit EA, Priess JR . Actin-dependent cytoplasmic streaming in C. elegans oogenesis. Development 2007; 134: 2227–2236.
Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO . Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 1999; 126: 1011–1022.
Hubbard EJA, Greenstein D . Introduction to the germ line. WormBook 2005 (http://www.wormbook.org).
Boag PR, Nakamura A, Blackwell TK . A conserved RNA–protein complex component involved in physiological germline apoptosis regulation in C. elegans. Development 2005; 132: 4975–4986.
Salinas LS, Maldonado E, Navarro RE . Stress-induced germ cell apoptosis by a p53 independent pathway in Caenorhabditis elegans. Cell Death Differ 2006; 13: 2129–2139.
Ito S, Greiss S, Gartner A, Derry WB . Cell-nonautonomous regulation of C. elegans germ cell death by kri-1. Curr Biol 2010; 20: 333–338.
Schertel C, Conradt B . C. elegans orthologs of components of the RB tumor suppressor complex have distinct pro-apoptotic functions. Development 2007; 134: 3691–3701.
Sendoel A, Kohler I, Fellmann C, Lowe SW, Hengartner MO . HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature 2010; 465: 577–583.
Reinke V, Gil IS, Ward S, Kazmer K . Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development 2004; 131: 311–323.
Wang X, Roy PJ, Holland SJ, Zhang LW, Culotti JG, Pawson T . Multiple ephrins control cell organization in C. elegans using kinase-dependent and -independent functions of the VAB-1 Eph receptor. Mol Cell 1999; 4: 903–913.
Schumacher B, Schertel C, Wittenburg N, Tuck S, Mitani S, Gartner A et al. C. elegans ced-13 can promote apoptosis and is induced in response to DNA damage. Cell Death Differ 2005; 12: 153–161.
Chin-Sang ID, Moseley SL, Ding M, Harrington RJ, George SE, Chisholm AD . The divergent C. elegans ephrin EFN-4 functions in embryonic morphogenesis in a pathway independent of the VAB-1 Eph receptor. Development 2002; 129: 5499–5510.
Hahn AC, Emmons SW . The roles of an ephrin and a semaphorin in patterning cell–cell contacts in C. elegans sensory organ development. Dev Biol 2003; 256: 379–388.
Cheng H, Govindan JA, Greenstein D . Regulated trafficking of the MSP/Eph receptor during oocyte meiotic maturation in C. elegans. Curr Biol 2008; 18: 705–714.
Andux S, Ellis RE . Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet 2008; 4: e1000295.
Luo S, Kleemann GA, Ashraf JM, Shaw WM, Murphy CT . TGF-beta and insulin signaling regulate reproductive aging via oocyte and germline quality maintenance. Cell 2010; 143: 299–312.
Schumacher B, Hofmann K, Boulton S, Gartner A . The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr Biol 2001; 11: 1722–1727.
Derry WB, Putzke AP, Rothman JH . Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 2001; 294: 591–595.
Sundaram MV . RTK/Ras/MAPK signaling. WormBook 2006 (http://www.wormbook.org).
Mohamed AM, Chin-Sang ID . Characterization of loss-of-function and gain-of-function Eph receptor tyrosine kinase signaling in C. elegans axon targeting and cell migration. Dev Biol 2006; 290: 164–176.
Hoeppner DJ, Hengartner MO, Schnabel R . Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature 2001; 412: 202–206.
Galvin BD, Kim S, Horvitz HR . Caenorhabditis elegans genes required for the engulfment of apoptotic corpses function in the cytotoxic cell deaths induced by mutations in lin-24 and lin-33. Genetics 2008; 179: 403–417.
Wang X, Li W, Zhao D, Liu B, Shi Y, Chen B et al. Caenorhabditis elegans transthyretin-like protein TTR-52 mediates recognition of apoptotic cells by the CED-1 phagocyte receptor. Nat Cell Biol 2010; 12: 655–664.
Angelo G, Van Gilst MR . Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science 2009; 326: 954–958.
Reddien PW, Cameron S, Horvitz HR . Phagocytosis promotes programmed cell death in C. elegans. Nature 2001; 412: 198–202.
Depaepe V, SuarezGonzalez N, Dufour A, Passante L, Gorski JA, Jones KR et al. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature 2005; 435: 1244–1250.
Pasquale EB . Eph–ephrin bidirectional signaling in physiology and disease. Cell 2008; 133: 38–52.
Sulston JE, Schierenberg E, White JG, Thomson JN . The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 1983; 100: 64–119.
Voutev R, Keating R, Hubbard EJA, Vallier LG . Characterization of the Caenorhabditis elegans islet LIM-homeodomain ortholog, lim-7. FEBS Lett 2009; 583: 456–464.
GengyoAndo K, Mitani S . Characterization of mutations induced by ethyl methanesulfonate, UV, and trimethylpsoralen in the nematode Caenorhabditis elegans. Biochem Biophys Res Commun 2000; 269: 64–69.
Strome S, Powers J, Dunn M, Reese K, Malone CJ, White J et al. Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos. Mol Biol Cell 2001; 12: 1751–1764.
Lee M, Schedl T . RNA in situ hybridization of dissected gonads. WormBook 2006 (http://www.wormbook.org).
Timmons L, Court DL, Fire A . Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 2001; 263: 103–112.
Kimble J . Alterations in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Dev Biol 1981; 87: 286–300.
Acknowledgements
We thank Hongtao Jia for helpful discussions and preliminary experiments, and Steven Billups, Mark Corkins, and Elizabeth Ulm for comments on the manuscript. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). Work in the Chamberlin lab is supported by the National Science Foundation (NSF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by E Baehrecke
Rights and permissions
About this article
Cite this article
Li, X., Johnson, R., Park, D. et al. Somatic gonad sheath cells and Eph receptor signaling promote germ-cell death in C. elegans. Cell Death Differ 19, 1080–1089 (2012). https://doi.org/10.1038/cdd.2011.192
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2011.192
Keywords
This article is cited by
-
Mitochondrial maturation drives germline stem cell differentiation in Caenorhabditis elegans
Cell Death & Differentiation (2020)
-
MiR-35 buffers apoptosis thresholds in the C. elegans germline by antagonizing both MAPK and core apoptosis pathways
Cell Death & Differentiation (2019)
-
Your neighbours matter – non-autonomous control of apoptosis in development and disease
Cell Death & Differentiation (2016)
-
EphA/ephrin-A signaling is critically involved in region-specific apoptosis during early brain development
Cell Death & Differentiation (2013)
-
Cell-nonautonomous inhibition of radiation-induced apoptosis by dynein light chain 1 in Caenorhabditis elegans
Cell Death & Disease (2013)