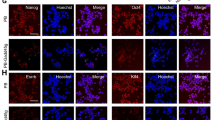
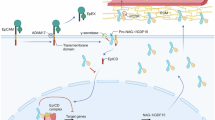
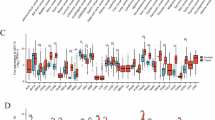
Abstract
Conversion of intestinal stem cells into tumor-initiating cells is an early step in ApcMin-induced polyposis. Wild-type p53-induced phosphatase 1 (Wip1)-dependent activation of a DNA damage response and p53 has a permanent role in suppression of stem cell conversion, and deletion of Wip1 lowers the tumor burden in ApcMin mice. Here we show that cyclin-dependent kinase inhibitor 2a, checkpoint kinase 2, and growth arrest and DNA damage gene 45a (Gadd45a) exert critical functions in the tumor-resistant phenotype of Wip1-deficient mice. We further identified Gadd45a as a haploinsufficient gene in the regulation of Wip1-dependent tumor resistance in mice. Gadd45a appears to function through its ability to activate the Jnk-dependent signaling pathway that in turn is a necessary mediator of the proapoptotic functions of p53 that respond to activation of the β-catenin signaling pathway. We propose that silencing of Gadd45a is sufficient to override p53 activation in the presence of active β-catenin under conditions of an enhanced DNA damage response.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- Wip1:
-
wild-type p53-induced phosphatase 1
- ATM:
-
ataxia telangiectasia mutated
- APC:
-
adenomatous polyposis coli
- gadd45a:
-
growth arrest and DNA damage gene 45a
- Cdkn2a:
-
cyclin-dependent kinase inhibitor 2a
- Chk2:
-
checkpoint kinase 2
- Lgr5:
-
leucine-rich repeat-containing G-protein coupled receptor 5
- IR:
-
ionizing irradiation
- GSK3:
-
Glycogen synthase kinase 3
- Mapk:
-
mitogen-activated protein kinase
- MKK7:
-
mitogen-activated protein kinase kinase 7
- IRES:
-
internal ribosome entry site
References
Pardal R, Clarke MF, Morrison SJ . Applying the principles of stem-cell biology to cancer. Nat Rev Cancer 2003; 3: 895–902.
Reya T, Morrison SJ, Clarke MF, Weissman IL . Stem cells, cancer, and cancer stem cells. Nature 2001; 414: 105–111.
Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009; 457: 608–611.
Pietsch EC, Sykes SM, McMahon SB, Murphy ME . The p53 family and programmed cell death. Oncogene 2008; 27: 6507–6521.
Horn HF, Vousden KH . Coping with stress: multiple ways to activate p53. Oncogene 2007; 26: 1306–1316.
Demidov ON, Timofeev O, Lwin HN, Kek C, Appella E, Bulavin DV . Wip1 phosphatase regulates p53-dependent apoptosis of stem cells and tumorigenesis in the mouse intestine. Cell Stem Cell 2007; 1: 180–190.
Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006; 444: 633–637.
Shreeram S, Hee WK, Demidov ON, Kek C, Yamaguchi H, Fornace AJ et al. Regulation of ATM/p53-dependent suppression of myc-induced lymphomas by Wip1 phosphatase. J Exp Med 2006; 203: 2793–2799.
Fujimoto H, Onishi N, Kato N, Takekawa M, Xu XZ, Kosugi A et al. Regulation of the antioncogenic Chk2 kinase by the oncogenic Wip1 phosphatase. Cell Death Differ 2006; 13: 1170–1180.
Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet 2002; 31: 210–215.
Li J, Yang Y, Peng Y, Austin RJ, van Eyndhoven WG, Nguyen KC et al. Oncogenic properties of PPM1D located within a breast cancer amplification epicenter at 17q23. Nat Genet 2002; 31: 133–134.
Bulavin DV, Phillips C, Nannenga B, Timofeev O, Donehower LA, Anderson CW et al. Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nat Genet 2004; 36: 343–350.
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449: 1003–1007.
Sangiorgi E, Capecchi MR . Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 2008; 40: 915–920.
Salic A, Lee E, Mayer L, Kirschner MW . Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell 2000; 5: 523–532.
Shreeram S, Demidov ON, Hee WK, Yamaguchi H, Onishi N, Kek C et al. Wip1 phosphatase modulates ATM-dependent signaling pathways. Mol Cell 2006; 23: 757–764.
Damalas A, Ben-Ze'ev A, Simcha I, Shtutman M, Leal JF, Zhurinsky J et al. Excess beta-catenin promotes accumulation of transcriptionally active p53. EMBO J 1999; 18: 3054–3063.
Wong ES, Le Guezennec X, Demidov ON, Marshall NT, Wang ST, Krishnamurthy J et al. p38MAPK controls expression of multiple cell cycle inhibitors and islet proliferation with advancing age. Dev Cell 2009; 17: 142–149.
Bulavin DV, Kovalsky O, Hollander MC, Fornace AJ . Loss of oncogenic H-ras-induced cell cycle arrest and p38 mitogen-activated protein kinase activation by disruption of Gadd45a. Mol Cell Biol 2003; 23: 3859–3871.
Hildesheim J, Bulavin DV, Anver MR, Alvord WG, Hollander MC, Vardanian L et al. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res 2002; 62: 7305–7315.
Takekawa M, Saito H . A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 1998; 95: 521–530.
Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 2006; 12: 446–451.
Takekawa M, Adachi M, Nakahata A, Nakayama I, Itoh F, Tsukuda H et al. p53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J 2000; 19: 6517–6526.
Matsuoka S, Huang M, Elledge SJ . Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 1998; 282: 1893–1897.
Takai H, Naka K, Okada Y, Watanabe M, Harada N, Saito S et al. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J 2002; 21: 5195–5205.
Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev 2004; 18: 1385–1390.
Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005; 434: 864–870.
Efeyan A, Serrano M . p53: guardian of the genome and policeman of the oncogenes. Cell Cycle 2007; 6: 1006–1010.
Tront JS, Hoffman B, Liebermann DA . Gadd45a suppresses Ras-driven mammary tumorigenesis by activation of c-Jun NH2-terminal kinase and p38 stress signaling resulting in apoptosis and senescence. Cancer Res 2006; 66: 8448–8454.
Schramek D, Kotsinas A, Meixner A, Wada T, Elling U, Pospisilik JA et al. The stress kinase MKK7 couples oncogenic stress to p53 stability and tumor suppression. Nat Genet 2011; 43: 212–219.
Bartek J, Bartkova J, Lukas J . DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene 2007; 26: 7773–7779.
Bowen TJ, Yakushiji H, Montagna C, Jain S, Ried T, Wynshaw-Boris A . Atm heterozygosity cooperates with loss of Brca1 to increase the severity of mammary gland cancer and reduce ductal branching. Cancer Res 2005; 65: 8736–8746.
Kinzler KW, Vogelstein B . Lessons from hereditary colorectal cancer. Cell 1996; 87: 159–170.
Mendrysa SM, O'Leary KA, McElwee MK, Michalowski J, Eisenman RN, Powell DA et al. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev 2006; 20: 16–21.
Tront JS, Huang Y, Fornace AJ, Hoffman B, Liebermann DA . Gadd45a functions as a promoter or suppressor of breast cancer dependent on the oncogenic stress. Cancer Res 2010; 70: 9671–9681.
Nateri AS, Spencer-Dene B, Behrens A . Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 2005; 437: 281–285.
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 2006; 22: 159–168.
Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res 2004; 64: 4394–4399.
Nutley BP, Smith NF, Hayes A, Kelland LR, Brunton L, Golding BT et al. Preclinical pharmacokinetics and metabolism of a novel prototype DNA-PK inhibitor NU7026. Br J Cancer 2005; 93: 1011–1018.
Acknowledgements
The research for DVB was supported by the Agency for Science, Technology and Research (Singapore) and for OND partially by Conseil Regional de Bourgogne. We are grateful to Dr. Penninger for providing an MKK7 vector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Oren
Rights and permissions
About this article
Cite this article
Demidov, O., Zhu, Y., Kek, C. et al. Role of Gadd45a in Wip1-dependent regulation of intestinal tumorigenesis. Cell Death Differ 19, 1761–1768 (2012). https://doi.org/10.1038/cdd.2012.57
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2012.57
Keywords
This article is cited by
-
Truncated PPM1D impairs stem cell response to genotoxic stress and promotes growth of APC-deficient tumors in the mouse colon
Cell Death & Disease (2019)
-
Wee1 inhibition potentiates Wip1-dependent p53-negative tumor cell death during chemotherapy
Cell Death & Disease (2016)