Abstract
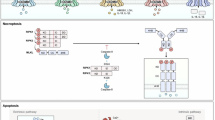
The thymus is the primary organ responsible for de novo generation of immunocompetent T cells that have a diverse repertoire of antigen recognition. During the developmental process, 98% of thymocytes die by apoptosis. Thus apoptosis is a dominant process in the thymus and occurs through either death by neglect or negative selection or through induction by stress/aging. Caspase activation is an essential part of the general apoptosis mechanism, and data suggest that caspases may have a role in negative selection; however, it seems more probable that caspase-8 activation is involved in death by neglect, particularly in glucocorticoid-induced thymocyte apoptosis. Caspase-8 is active in double-positive (DP) thymocytes in vivo and can be activated in vitro in DP thymocytes by T-cell receptor (TCR) crosslinking to induce apoptosis. Caspase-8 is a proapoptotic member of the caspase family and is considered an initiator caspase, which is activated upon stimulation of a death receptor (e.g., Fas), recruitment of the adaptor molecule FADD, and recruitment and subsequent processing of procaspase-8. The main role of caspase-8 seems to be pro-apoptotic and, in this review, we will discuss about the involvement of caspase-8 in (1) TCR-triggered thymic apoptosis; (2) death receptor-mediated thymic apoptosis; and (3) glucocorticoid-induced thymic apoptosis. Regarding TCR triggering, caspase-8 is active in medullary, semi-mature heat-stable antigenhi (HAShi SP) thymocytes as a consequence of strong TCR stimulation. The death receptors Fas, FADD, and FLIP are involved upstream of caspase-8 activation in apoptosis; whereas, Bid and HDAC7 are involved downstream of caspase-8. Finally, caspase-8 is involved in glucocortocoid-induced thymocyte apoptosis through an activation loop with the protein GILZ. GILZ activates caspase-8, promoting GILZ sumoylation and its protection from proteasomal degradation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- TEC:
-
thymic epithelial cell
- DP:
-
double positive
- TCR:
-
T-cell receptor
- mTEC:
-
medullary TEC
- DED:
-
death effector domain
- DISC:
-
death-inducing signaling complex
- cFLIP:
-
cellular FLICE inhibitory protein
- IAP:
-
inhibitor of apoptosis
- AIF:
-
apoptosis-inducing factor
- SP:
-
single positive
- NOD:
-
non-obese diabetic
- FAIM:
-
Fas apoptosis inhibitory molecule
- HDAC7:
-
histone deacetylase 7
- GC:
-
glucocorticoid
- GR:
-
GC receptor
- DEX:
-
dexamethasone
- MPS:
-
methylprednisolone
References
Shanley DP, Aw D, Manley NR, Palmer DB . An evolutionary perspective on the mechanisms of immunosenescence. Trends immunol 2009; 30: 374–381.
Gui J, Mustachio LM, Su DM, Craig RW . Thymus size and age-related thymic involution: early programming, sexual dimorphism, progenitors and stroma. Aging Dis 2012; 3: 280–290.
Shortman K, Egerton M, Spangrude GJ, Scollay R . The generation and fate of thymocytes. Semin Immunol 1990; 2: 3–12.
Surh CD, Sprent J . T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature 1994; 372: 100–103.
Janaway CA Jr, Travers P, Walport M, Shlomchik MJ . The development and survival of lymphocytes. In: Immunobiology, the immune system in health and disease Garland Science Publishing: New York, 2005 pp 251–257.
Godfrey DI, Purton JF, Boyd RL, Cole TJ . Stress-free T-cell development: glucocorticoids are not obligatory. Immunol Today 2000; 21: 606–611.
Alam SM, Davies GM, Lin CM, Zal T, Nasholds W, Jameson SC et al. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity 1999; 10: 227–237.
Gronski MA, Boulter JM, Moskophidis D, Nguyen LT, Holmberg K, Elford AR et al. TCR affinity and negative regulation limit autoimmunity. Nat Med 2004; 10: 1234–1239.
Mace PD, Riedl SJ . Molecular cell death platforms and assemblies. Curr Opin Cell Biol 2010; 22: 828–836.
Scott FL, Stec B, Pop C, Dobaczewska MK, Lee JJ, Monosov E et al. The Fas-FADD death domain complex structure unravels signalling by receptor clustering. Nature 2009; 457: 1019–1022.
Wang L, Yang JK, Kabaleeswaran V, Rice AJ, Cruz AC, Park AY et al. The Fas-FADD death domain complex structure reveals the basis of DISC assembly and disease mutations. Nat Struct Mol Biol 2010; 17: 1324–1329.
Tu S, McStay GP, Boucher LM, Mak T, Beere HM, Green DR . In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nature Cell Biol 2006; 8: 72–77.
Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM et al. A unified model for apical caspase activation. Mol Cell 2003; 11: 529–541.
Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM . An induced proximity model for caspase-8 activation. J Biol Chem 1998; 273: 2926–2930.
Keller N, Mares J, Zerbe O, Grutter MG . Structural and biochemical studies on procaspase-8: new insights on initiator caspase activation. Structure 2009; 17: 438–448.
Keller N, Grutter MG, Zerbe O . Studies of the molecular mechanism of caspase-8 activation by solution NMR. Cell Death Differ 2010; 17: 710–718.
Oberst A, Pop C, Tremblay AG, Blais V, Denault JB, Salvesen GS et al. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J Biol Chem 2010; 285: 16632–16642.
Pop C, Fitzgerald P, Green DR, Salvesen GS . Role of proteolysis in caspase-8 activation and stabilization. Biochemistry 2007; 46: 4398–4407.
Pop C, Oberst A, Drag M, Van Raam BJ, Riedl SJ, Green DR et al. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem J 2011; 433: 447–457.
Chang DW, Xing Z, Capacio VL, Peter ME, Yang X . Interdimer processing mechanism of procaspase-8 activation. EMBO J 2003; 22: 4132–4142.
Donepudi M, Mac Sweeney A, Briand C, Grutter MG . Insights into the regulatory mechanism for caspase-8 activation. Mol Cell 2003; 11: 543–549.
Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH et al. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J 1997; 16: 2794–2804.
Hughes MA, Harper N, Butterworth M, Cain K, Cohen GM, MacFarlane M . Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Mol Cell 2009; 35: 265–279.
Kantari C, Walczak H . Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta 2011; 1813: 558–563.
Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 2008; 4: 313–321.
Declercq W, Vanden Berghe T, Vandenabeele P . RIP kinases at the crossroads of cell death and survival. Cell 2009; 138: 229–232.
van Raam BJ, Salvesen GS . Proliferative versus apoptotic functions of caspase-8 Hetero or homo: the caspase-8 dimer controls cell fate. Biochim Biophys Acta 2012; 1824: 113–122.
Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 1998; 9: 267–276.
Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 1998; 279: 1954–1958.
Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity 2000; 12: 633–642.
Leverrier S, Salvesen GS, Walsh CM . Enzymatically active single chain caspase-8 maintains T-cell survival during clonal expansion. Cell Death Differ 2011; 18: 90–98.
Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 2002; 419: 395–399.
Clayton LK, Ghendler Y, Mizoguchi E, Patch RJ, Ocain TD, Orth K et al. T-cell receptor ligation by peptide/MHC induces activation of a caspase in immature thymocytes: the molecular basis of negative selection. EMBO J 1997; 16: 2282–2293.
Hara H, Takeda A, Takeuchi M, Wakeham AC, Itie A, Sasaki M et al. The apoptotic protease-activating factor 1-mediated pathway of apoptosis is dispensable for negative selection of thymocytes. J Immunol 2002; 168: 2288–2295.
Doerfler P, Forbush KA, Perlmutter RM . Caspase enzyme activity is not essential for apoptosis during thymocyte development. J Immunol 2000; 164: 4071–4079.
Izquierdo M, Grandien A, Criado LM, Robles S, Leonardo E, Albar JP et al. Blocked negative selection of developing T cells in mice expressing the baculovirus p35 caspase inhibitor. EMBO J 1999; 18: 156–166.
Robles MS, Leonardo E, Criado LM, Izquierdo M, Martinez AC . Inhibitor of apoptosis protein from Orgyia pseudotsugata nuclear polyhedrosis virus provides a costimulatory signal required for optimal proliferation of developing thymocytes. J Immunol 2002; 168: 1770–1779.
Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 2001; 410: 549–554.
Li LY, Luo X, Wang X . Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001; 412: 95–99.
Delfino DV, Pozzesi N, Pierangeli S, Ayroldi E, Fierabracci A . Manipulating thymic apoptosis for future therapy of autoimmune diseases. Curr Pharm Des 2011; 17: 3108–3119.
Jiang D, Zheng L, Lenardo MJ . Caspases in T-cell receptor-induced thymocyte apoptosis. Cell Death Differ 1999; 6: 402–411.
Kishimoto H, Sprent J . A defect in central tolerance in NOD mice. Nat Immunol 2001; 2: 1025–1031.
Kishimoto H, Surh CD, Sprent J . A role for Fas in negative selection of thymocytes in vivo . J Exp Med 1998; 187: 1427–1438.
OhYama T, Tsukumo S, Yajima N, Sakamaki K, Yonehara S . Reduction of thymocyte numbers in transgenic mice expressing viral FLICE-inhibitory protein in a Fas-independent manner. Microbiol Immunol 2000; 44: 289–297.
Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 2011; 471: 363–367.
Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 2011; 471: 368–372.
Zhong X, Schneider TJ, Cabral DS, Donohoe TJ, Rothstein TL . An alternatively spliced long form of Fas apoptosis inhibitory molecule (FAIM) with tissue-specific expression in the brain. Mol Immunol 2001; 38: 65–72.
Huo J, Xu S, Lam KP . Fas apoptosis inhibitory molecule regulates T cell receptor-mediated apoptosis of thymocytes by modulating Akt activation and Nur77 expression. J Biol Chem 2010; 285: 11827–11835.
Nomura J, Matsumoto K, Iguchi-Ariga SM, Ariga H . Mitochondria-independent induction of Fas-mediated apoptosis by MSSP. Oncol Rep 2005; 14: 1305–1309.
Hueber AO, Bernard AM, Herincs Z, Couzinet A, He HT . An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes. EMBO Rep 2002; 3: 190–196.
Zhang J, Cado D, Chen A, Kabra NH, Winoto A . Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 1998; 392: 296–300.
Rosenberg S, Zhang H, Zhang J . FADD deficiency impairs early hematopoiesis in the bone marrow. J Immunol 2011; 186: 203–213.
Oehme I, Neumann F, Bosser S, Zornig M . Transgenic overexpression of the Caspase-8 inhibitor FLIP(short) leads to impaired T cell proliferation and an increased memory T cell pool after staphylococcal enterotoxin B injection. Eur J Immunol 2005; 35: 1240–1249.
Cretney E, Uldrich AP, Berzins SP, Strasser A, Godfrey DI, Smyth MJ . Normal thymocyte negative selection in TRAIL-deficient mice. J Exp Med 2003; 198: 491–496.
Couzinet A, Tamura K, Chen HM, Nishimura K, Wang Z, Morishita Y et al. A cell-type-specific requirement for IFN regulatory factor 5 (IRF5) in Fas-induced apoptosis. Proc Natl Acad Sci USA 2008; 105: 2556–2561.
Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 1999; 400: 886–891.
Scott FL, Fuchs GJ, Boyd SE, Denault JB, Hawkins CJ, Dequiedt F et al. Caspase-8 cleaves histone deacetylase 7 and abolishes its transcription repressor function. J Biol Chem 2008; 283: 19499–19510.
He YW . Orphan nuclear receptors in T lymphocyte development. J Leuk Biol 2002; 72: 440–446.
Siggs OM, Makaroff LE, Liston A . The why and how of thymocyte negative selection. Curr Opin Immunol 2006; 18: 175–183.
Dequiedt F, Kasler H, Fischle W, Kiermer V, Weinstein M, Herndier BG et al. HDAC7, a thymus-specific class II histone deacetylase, regulates Nur77 transcription and TCR-mediated apoptosis. Immunity 2003; 18: 687–698.
Parra M, Kasler H, McKinsey TA, Olson EN, Verdin E . Protein kinase D1 phosphorylates HDAC7 and induces its nuclear export after T-cell receptor activation. J Biol Chem 2005; 280: 13762–13770.
Li X, Song S, Liu Y, Ko SH, Kao HY . Phosphorylation of the histone deacetylase 7 modulates its stability and association with 14-3-3 proteins. J Biol Chem 2004; 279: 34201–34208.
Ashwell JD, Lu FW, Vacchio MS . Glucocorticoids in T cell development and function*. Annu Rev Immunol 2000; 18: 309–345.
Mann CL, Bortner CD, Jewell CM, Cidlowski JA . Glucocorticoid-induced plasma membrane depolarization during thymocyte apoptosis: association with cell shrinkage and degradation of the Na(+)/K(+)-adenosine triphosphatase. Endocrinology 2001; 142: 5059–5068.
Bustamante J, Bersier G, Badin RA, Cymeryng C, Parodi A, Boveris A . Sequential NO production by mitochondria and endoplasmic reticulum during induced apoptosis. Nitric Oxide 2002; 6: 333–341.
Marchetti MC, Di Marco B, Cifone G, Migliorati G, Riccardi C . Dexamethasone-induced apoptosis of thymocytes: role of glucocorticoid receptor-associated Src kinase and caspase-8 activation. Blood 2003; 101: 585–593.
Lepine S, Lakatos B, Courageot MP, Le Stunff H, Sulpice JC, Giraud F . Sphingosine contributes to glucocorticoid-induced apoptosis of thymocytes independently of the mitochondrial pathway. J Immunol 2004; 173: 3783–3790.
D'Adamio F, Zollo O, Moraca R, Ayroldi E, Bruscoli S, Bartoli A et al. A new dexamethasone-induced gene of the leucine zipper family protects T lymphocytes from TCR/CD3-activated cell death. Immunity 1997; 7: 803–812.
Ayroldi E, Migliorati G, Bruscoli S, Marchetti C, Zollo O, Cannarile L et al. Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor kappaB. Blood 2001; 98: 743–753.
Delfino DV, Agostini M, Spinicelli S, Vito P, Riccardi C . Decrease of Bcl-xL and augmentation of thymocyte apoptosis in GILZ overexpressing transgenic mice. Blood 2004; 104: 4134–4141.
Ayroldi E, Zollo O, Bastianelli A, Marchetti C, Agostini M, Di Virgilio R et al. GILZ mediates the antiproliferative activity of glucocorticoids by negative regulation of Ras signaling. J Clin Invest 2007; 117: 1605–1615.
Cannarile L, Fallarino F, Agostini M, Cuzzocrea S, Mazzon E, Vacca C et al. Increased GILZ expression in transgenic mice up-regulates Th-2 lymphokines. Blood 2006; 107: 1039–1047.
Cannarile L, Cuzzocrea S, Santucci L, Agostini M, Mazzon E, Esposito E et al. Glucocorticoid-induced leucine zipper is protective in Th1-mediated models of colitis. Gastroenterology 2009; 136: 530–541.
Cohen N, Mouly E, Hamdi H, Maillot MC, Pallardy M, Godot V et al. GILZ expression in human dendritic cells redirects their maturation and prevents antigen-specific T lymphocyte response. Blood 2006; 107: 2037–2044.
Hamdi H, Godot V, Maillot MC, Prejean MV, Cohen N, Krzysiek R et al. Induction of antigen-specific regulatory T lymphocytes by human dendritic cells expressing the glucocorticoid-induced leucine zipper. Blood 2007; 110: 211–219.
Muller OG, Parnova RG, Centeno G, Rossier BC, Firsov D, Horisberger JD . Mineralocorticoid effects in the kidney: correlation between alphaENaC, GILZ, and Sgk-1 mRNA expression and urinary excretion of Na+ and K+. J Am Soc Nephrol 2003; 14: 1107–1115.
Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D . A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem 2005; 280: 39970–39981.
Ayroldi E, Riccardi C . Glucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid action. FASEB J 2009; 23: 3649–3658.
Ayroldi E, Cannarile L, Migliorati G, Nocentini G, Delfino DV, Riccardi C . Mechanisms of the anti-inflammatory effects of glucocorticoids: genomic and nongenomic interference with MAPK signaling pathways. FASEB J 2012; 26: 4805–4820.
Delfino DV, Spinicelli S, Pozzesi N, Pierangeli S, Velardi E, Bruscoli S et al. Glucocorticoid-induced activation of caspase-8 protects the glucocorticoid-induced protein Gilz from proteasomal degradation and induces its binding to SUMO-1 in murine thymocytes. Cell Death Differ 2011; 18: 183–190.
Burns TF, Bernhard EJ, El-Deiry WS . Tissue specific expression of p53 target genes suggests a key role for KILLER/DR5 in p53-dependent apoptosis in vivo . Oncogene 2001; 20: 4601–4612.
Bruscoli S, Di Virgilio R, Donato V, Velardi E, Baldoni M, Marchetti C et al. Genomic and non-genomic effects of different glucocorticoids on mouse thymocyte apoptosis. Eur J Pharmacol 2006; 529: 63–70.
Wang D, Muller N, McPherson KG, Reichardt HM . Glucocorticoids engage different signal transduction pathways to induce apoptosis in thymocytes and mature T cells. J Immunol 2006; 176: 1695–1702.
Farias-de-Oliveira DA, Villa-Verde DM, Nunes Panzenhagen PH, Silva dos Santos D, Berbert LR, Savino W et al. Caspase-8 and caspase-9 mediate thymocyte apoptosis in Trypanosoma cruzi acutely infected mice. J Leuk Biol 2013; 93: 227–234.
Bidere N, Su HC, Lenardo MJ . Genetic disorders of programmed cell death in the immune system. Annu Rev Immunol 2006; 24: 321–352.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by J Cidlowski
Rights and permissions
About this article
Cite this article
Pozzesi, N., Fierabracci, A., Liberati, A. et al. Role of caspase-8 in thymus function. Cell Death Differ 21, 226–233 (2014). https://doi.org/10.1038/cdd.2013.166
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2013.166
Keywords
This article is cited by
-
Synthesize of Bi2O3/Gln-TSC nanoparticles and evaluation of their toxicity on prostate cancer cells and expression of CASP8, BAX, and Bcl-2 genes
Scientific Reports (2022)
-
Sex differences of inflammatory and immune response in pups of Wistar rats with SIRS
Scientific Reports (2020)
-
FasL-PDPK1 Pathway Promotes the Cytotoxicity of CD8+ T Cells During Ischemic Stroke
Translational Stroke Research (2020)
-
Maternal Glucocorticoid Elevation and Associated Fetal Thymocyte Apoptosis are Involved in Immune Disorders of Prenatal Caffeine Exposed Offspring Mice
Scientific Reports (2017)
-
Non-apoptotic functions of caspases in myeloid cell differentiation
Cell Death & Differentiation (2017)