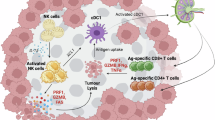
Abstract
Protection against cellular stress from various sources, such as nutritional, physical, pathogenic, or oncogenic, results in the induction of both intrinsic and extrinsic cellular protection mechanisms that collectively limit the damage these insults inflict on the host. The major extrinsic protection mechanism against cellular stress is the immune system. Indeed, it has been well described that cells that are stressed due to association with viral infection or early malignant transformation can be directly sensed by the immune system, particularly natural killer (NK) cells. Although the ability of NK cells to directly recognize and respond to stressed cells is well appreciated, the mechanisms and the breadth of cell-intrinsic responses that are intimately linked with their activation are only beginning to be uncovered. This review will provide a brief introduction to NK cells and the relevant receptors and ligands involved in direct responses to cellular stress. This will be followed by an in-depth discussion surrounding the various intrinsic responses to stress that can naturally engage NK cells, and how therapeutic agents may induce specific activation of NK cells and other innate immune cells by activating cellular responses to stress.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- NK:
-
natural killer
- NKG2D:
-
natural killer group 2D
- DNAM-1:
-
DNAX accessory molecule-1
- MHC:
-
major histocompatibility complex
- MIC:
-
MHC class I chain-related protein
- ULBP:
-
UL16-binding protein
- Rae:
-
retinoic acid early inducible
- ATM:
-
ataxia telangiectasia mutated
- ATR:
-
ATM and Rad3-related
- miRNA:
-
microRNA
- HSP:
-
heat-shock protein
References
Lowe SW, Cepero E, Evan G . Intrinsic tumour suppression. Nature 2004; 432: 307–315.
Griffith TS, Ferguson TA . Cell death in the maintenance and abrogation of tolerance: the five Ws of dying cells. Immunity 2011; 35: 456–466.
Raulet DH, Guerra N . Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev 2009; 9: 568–580.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L . Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31: 51–72.
Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28: 105–113.
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13: 1050–1059.
Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S . Functions of natural killer cells. Nat Immunol 2008; 9: 503–510.
Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA et al. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol 1999; 162: 6658–6662.
Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med 2000; 191: 661–668.
Smyth MJ, Crowe NY, Godfrey DI . NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol 2001; 13: 459–463.
Andoniou CE, Andrews DM, Degli-Esposti MA . Natural killer cells in viral infection: more than just killers. Immunol Rev 2006; 214: 239–250.
Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA . Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol 2003; 4: 175–181.
Raulet DH, Vance RE . Self-tolerance of natural killer cells. Nat Rev 2006; 6: 520–531.
Karre K . Natural killer cell recognition of missing self. Nat Immunol 2008; 9: 477–480.
Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M . NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol 2002; 3: 1150–1155.
Vivier E, Nunes JA, Vely F . Natural killer cell signaling pathways. Science 2004; 306: 1517–1519.
Campbell JL, Klueva NY, Zheng HG, Nieto-Sotelo J, Ho TD, Nguyen HT . Cloning of new members of heat shock protein HSP101 gene family in wheat (Triticum aestivum (L.) Moench) inducible by heat, dehydration, and ABA(1). Biochim Biophys Acta 2001; 1517: 270–277.
Purdy AK, Campbell KS . SHP-2 expression negatively regulates NK cell function. J Immunol 2009; 183: 7234–7243.
Yusa S, Catina TL, Campbell KS . SHP-1- and phosphotyrosine-independent inhibitory signaling by a killer cell Ig-like receptor cytoplasmic domain in human NK cells. J Immunol 2002; 168: 5047–5057.
Yusa S, Campbell KS . Src homology region 2-containing protein tyrosine phosphatase-2 (SHP-2) can play a direct role in the inhibitory function of killer cell Ig-like receptors in human NK cells. J Immunol 2003; 170: 4539–4547.
Lanier LL . Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9: 495–502.
Raulet DH . Roles of the NKG2D immunoreceptor and its ligands. Nat Rev 2003; 3: 781–790.
Champsaur M, Lanier LL . Effect of NKG2D ligand expression on host immune responses. Immunol Rev 2010; 235: 267–285.
Raulet DH, Gasser S, Gowen BG, Deng W, Jung H . Regulation of Ligands for the NKG2D Activating Receptor. Annu Rev Immunol 2013; 31: 413–441.
Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999; 285: 727–729.
Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 2001; 14: 123–133.
Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 2000; 12: 721–727.
Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM . Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J Immunol 2002; 169: 4079–4083.
Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH . Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol 2000; 1: 119–126.
Cerwenka A, Lanier LL . Natural killer cells, viruses and cancer. Nat Rev 2001; 1: 41–49.
Pende D, Cantoni C, Rivera P, Vitale M, Castriconi R, Marcenaro S et al. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol 2001; 31: 1076–1086.
Diefenbach A, Jensen ER, Jamieson AM, Raulet DH . Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 2001; 413: 165–171.
Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH . The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity 2002; 17: 19–29.
Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T . Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA 1999; 96: 6879–6884.
Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR . Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J Clin Investig 2004; 114: 560–568.
Hayashi T, Imai K, Morishita Y, Hayashi I, Kusunoki Y, Nakachi K . Identification of the NKG2D haplotypes associated with natural cytotoxic activity of peripheral blood lymphocytes and cancer immunosurveillance. Cancer Res 2006; 66: 563–570.
Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y . NKG2D function protects the host from tumor initiation. J Exp Med 2005; 202: 583–588.
Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008; 28: 571–580.
Groh V, Wu J, Yee C, Spies T . Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002; 419: 734–738.
Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J et al. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci USA 2008; 105: 1285–1290.
Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 1996; 4: 573–581.
Fuchs A, Colonna M . The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin Cancer Biol 2006; 16: 359–366.
Chan CJ, Andrews DM, Smyth MJ . Receptors that interact with nectin and nectin-like proteins in the immunosurveillance and immunotherapy of cancer. Curr Opin Immunol 2012; 24: 246–251.
Sherrington PD, Scott JL, Jin B, Simmons D, Dorahy DJ, Lloyd J et al. TLiSA1 (PTA1) activation antigen implicated in T cell differentiation and platelet activation is a member of the immunoglobulin superfamily exhibiting distinctive regulation of expression. J Biol Chem 1997; 272: 21735–21744.
Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med 2003; 198: 557–567.
Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112). Blood 2005; 105: 2066–2073.
Gilfillan S, Chan CJ, Cella M, Haynes NM, Rapaport AS, Boles KS et al. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med 2008; 205: 2965–2973.
Iguchi-Manaka A, Kai H, Yamashita Y, Shibata K, Tahara-Hanaoka S, Honda S et al. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J Exp Med 2008; 205: 2959–2964.
Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Investig 2009; 119: 1251–1263.
Chan CJ, Andrews DM, McLaughlin NM, Yagita H, Gilfillan S, Colonna M et al. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J Immunol 2010; 184: 902–911.
Tomasec P, Wang EC, Davison AJ, Vojtesek B, Armstrong M, Griffin C et al. Downregulation of natural killer cell-activating ligand CD155 by human cytomegalovirus UL141. Nat Immunol 2005; 6: 181–188.
Morisaki T, Onishi H, Katano M . Cancer immunotherapy using NKG2D and DNAM-1 systems. Anticancer Res 2012; 32: 2241–2247.
Pende D, Bottino C, Castriconi R, Cantoni C, Marcenaro S, Rivera P et al. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis. Mol Immunol 2005; 42: 463–469.
El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res 2007; 67: 8444–8449.
Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D . Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res 2010; 16: 3901–3909.
Mamessier E, Sylvain A, Bertucci F, Castellano R, Finetti P, Houvenaeghel G et al. Human breast tumor cells induce self-tolerance mechanisms to avoid NKG2D-mediated and DNAM-mediated NK cell recognition. Cancer Res 2011; 71: 6621–6632.
Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Investig 2011; 121: 3609–3622.
Croxford JL, Tang ML, Pan MF, Huang CW, Kamran N, Phua CM et al. ATM-dependent spontaneous regression of early Emu-myc-induced murine B cell leukemia depends on NK and T cells. Blood 2013; 121: 2512–2521.
Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006; 444: 633–637.
Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 2005; 434: 907–913.
Shiloh Y . ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 2003; 3: 155–168.
Gasser S, Raulet DH . The DNA damage response arouses the immune system. Cancer Res 2006; 66: 3959–3962.
Gasser S, Orsulic S, Brown EJ, Raulet DH . The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005; 436: 1186–1190.
Vales-Gomez M, Chisholm SE, Cassady-Cain RL, Roda-Navarro P, Reyburn HT . Selective induction of expression of a ligand for the NKG2D receptor by proteasome inhibitors. Cancer Res 2008; 68: 1546–1554.
Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood 2009; 113: 3503–3511.
Berghuis D, Schilham MW, Vos HI, Santos SJ, Kloess S, Buddingh EP et al. Histone deacetylase inhibitors enhance expression of NKG2D ligands in Ewing sarcoma and sensitize for natural killer cell-mediated cytolysis. Clin Sarcoma Res 2012; 2: 8.
Cerboni C, Zingoni A, Cippitelli M, Piccoli M, Frati L, Santoni A . Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood 2007; 110: 606–615.
Gourzi P, Leonova T, Papavasiliou FN . A role for activation-induced cytidine deaminase in the host response against a transforming retrovirus. Immunity 2006; 24: 779–786.
Norman JM, Mashiba M, McNamara LA, Onafuwa-Nuga A, Chiari-Fort E, Shen W et al. The antiviral factor APOBEC3G enhances the recognition of HIV-infected primary T cells by natural killer cells. Nat Immunol 2011; 12: 975–983.
Ardolino M, Zingoni A, Cerboni C, Cecere F, Soriani A, Iannitto ML et al. DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK-T cell interaction. Blood 2011; 117: 4778–4786.
Wu GS, Burns TF, McDonald ER 3rd, Jiang W, Meng R, Krantz ID et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet 1997; 17: 141–143.
Johnstone RW, Frew AJ, Smyth MJ . The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer 2008; 8: 782–798.
Narita M, Lowe SW . Senescence comes of age. Nat Med 2005; 11: 920–922.
Kuilman T, Michaloglou C, Mooi WJ, Peeper DS . The essence of senescence. Genes Dev 2010; 24: 2463–2479.
Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008; 133: 1019–1031.
Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008; 133: 1006–1018.
Krizhanovsky V, Xue W, Zender L, Yon M, Hernando E, Lowe SW . Implications of cellular senescence in tissue damage response, tumor suppression, and stem cell biology. Cold Spring Harb Symp Quant Biol 2008; 73: 513–522.
Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007; 445: 656–660.
Sagiv A, Biran A, Yon M, Simon J, Lowe SW, Krizhanovsky V . Granule exocytosis mediates immune surveillance of senescent cells. Oncogene 2012 e-pub ahead of print 2 July 2012; doi:10.1038/onc.2012.206.
Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C et al. Senescence of activated stellate cells limits liver fibrosis. Cell 2008; 134: 657–667.
Wall M, Poortinga G, Stanley KL, Lindemann RK, Bots M, Chan CJ et al. The mTORC1 inhibitor Everolimus prevents and treats Emu-Myc lymphoma by restoring oncogene-induced senescence. Cancer Discov 2013; 3: 82–95.
Poggi A, Pardi R, Pella N, Morelli L, Sivori S, Vitale M et al. CD45-mediated regulation of LFA1 function in human natural killer cells. Anti-CD45 monoclonal antibodies inhibit the calcium mobilization induced via LFA1 molecules. Eur J Immunol 1993; 23: 2454–2463.
Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A . Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 2007; 26: 503–517.
d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003; 426: 194–198.
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW . Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997; 88: 593–602.
Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 2006; 444: 638–642.
Green DR, Kroemer G . Cytoplasmic functions of the tumour suppressor p53. Nature 2009; 458: 1127–1130.
Rivas C, Aaronson SA, Munoz-Fontela C . Dual Role of p53 in Innate Antiviral Immunity. Viruses 2010; 2: 298–313.
Munoz-Fontela C, Garcia MA, Garcia-Cao I, Collado M, Arroyo J, Esteban M et al. Resistance to viral infection of super p53 mice. Oncogene 2005; 24: 3059–3062.
Turpin E, Luke K, Jones J, Tumpey T, Konan K, Schultz-Cherry S . Influenza virus infection increases p53 activity: role of p53 in cell death and viral replication. J Virol 2005; 79: 8802–8811.
Textor S, Fiegler N, Arnold A, Porgador A, Hofmann TG, Cerwenka A . Human NK cells are alerted to induction of p53 in cancer cells by upregulation of the NKG2D ligands ULBP1 and ULBP2. Cancer Res 2011; 71: 5998–6009.
Li H, Lakshmikanth T, Garofalo C, Enge M, Spinnler C, Anichini A et al. Pharmacological activation of p53 triggers anticancer innate immune response through induction of ULBP2. Cell Cycle 2011; 10: 3346–3358.
Heinemann A, Zhao F, Pechlivanis S, Eberle J, Steinle A, Diederichs S et al. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res 2012; 72: 460–471.
Munoz-Fontela C, Macip S, Martinez-Sobrido L, Brown L, Ashour J, Garcia-Sastre A et al. Transcriptional role of p53 in interferon-mediated antiviral immunity. J Exp Med 2008; 205: 1929–1938.
Imbeault M, Ouellet M, Tremblay MJ . Microarray study reveals that HIV-1 induces rapid type-I interferon-dependent p53 mRNA up-regulation in human primary CD4+ T cells. Retrovirology 2009; 6: 5.
Taura M, Eguma A, Suico MA, Shuto T, Koga T, Komatsu K et al. p53 regulates Toll-like receptor 3 expression and function in human epithelial cell lines. Mol Cell Biol 2008; 28: 6557–6567.
Unni AM, Bondar T, Medzhitov R . Intrinsic sensor of oncogenic transformation induces a signal for innate immunosurveillance. Proc Natl Acad Sci USA 2008; 105: 1686–1691.
Liu XV, Ho SS, Tan JJ, Kamran N, Gasser S . Ras activation induces expression of Raet1 family NK receptor ligands. J Immunol 2012; 189: 1826–1834.
Dang CV . MYC on the path to cancer. Cell 2012; 149: 22–35.
Tokuyama M, Lorin C, Delebecque F, Jung H, Raulet DH, Coscoy L . Expression of the RAE-1 family of stimulatory NK-cell ligands requires activation of the PI3K pathway during viral infection and transformation. PLoS Pathogens 2011; 7: e1002265.
Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC . The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Microbiol 2008; 6: 266–275.
Zhao L, Vogt PK, Class I . PI3K in oncogenic cellular transformation. Oncogene 2008; 27: 5486–5496.
Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N . eIF4E—from translation to transformation. Oncogene 2004; 23: 3172–3179.
Ruggero D . The role of Myc-induced protein synthesis in cancer. Cancer Res 2009; 69: 8839–8843.
Walsh D . Manipulation of the host translation initiation complex eIF4F by DNA viruses. Biochem Soc Trans 2010; 38: 1511–1516.
Hartl FU, Hayer-Hartl M . Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 2002; 295: 1852–1858.
Jolly C, Morimoto RI . Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst 2000; 92: 1564–1572.
Evans CG, Chang L, Gestwicki JE . Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem 2010; 53: 4585–4602.
Johnson JD, Fleshner M . Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol 2006; 79: 425–434.
Srivastava P . Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev 2002; 2: 185–194.
Udono H, Srivastava PK . Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med 1993; 178: 1391–1396.
Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H et al. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol 1999; 162: 3757–3760.
Srivastava P . Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol 2002; 20: 395–425.
Tsan MF, Gao B . Heat shock proteins and immune system. J Leukoc Biol 2009; 85: 905–910.
Liu B, Dai J, Zheng H, Stoilova D, Sun S, Li Z . Cell surface expression of an endoplasmic reticulum resident heat shock protein gp96 triggers MyD88-dependent systemic autoimmune diseases. Proc Natl Acad Sci USA 2003; 100: 15824–15829.
Zheng H, Dai J, Stoilova D, Li Z . Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J Immunol 2001; 167: 6731–6735.
Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R . Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol 1997; 158: 4341–4350.
Gastpar R, Gross C, Rossbacher L, Ellwart J, Riegger J, Multhoff G . The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK cells. J Immunol 2004; 172: 972–980.
Gross C, Hansch D, Gastpar R, Multhoff G . Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem 2003; 384: 267–279.
Elsner L, Muppala V, Gehrmann M, Lozano J, Malzahn D, Bickeboller H et al. The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J Immunol 2007; 179: 5523–5533.
Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 2005; 65: 5238–5247.
Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL et al. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 2012; 287: 15874–15885.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Lavia P, Mileo AM, Giordano A, Paggi MG . Emerging roles of DNA tumor viruses in cell proliferation: new insights into genomic instability. Oncogene 2003; 22: 6508–6516.
Jung H, Hsiung B, Pestal K, Procyk E, Raulet DH . RAE-1 ligands for the NKG2D receptor are regulated by E2F transcription factors, which control cell cycle entry. J Exp Med 2012; 209: 2409–2422.
Guo H, Ingolia NT, Weissman JS, Bartel DP . Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010; 466: 835–840.
Ambros V . The functions of animal microRNAs. Nature 2004; 431: 350–355.
Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N et al. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol 2008; 9: 1065–1073.
Tsukerman P, Stern-Ginossar N, Gur C, Glasner A, Nachmani D, Bauman Y et al. MiR-10b downregulates the stress-induced cell surface molecule MICB, a critical ligand for cancer cell recognition by natural killer cells. Cancer Res 2012; 72: 5463–5472.
Cho WC . OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer 2007; 6: 60.
Nice TJ, Coscoy L, Raulet DH . Posttranslational regulation of the NKG2D ligand Mult1 in response to cell stress. J Exp Med 2009; 206: 287–298.
Salih HR, Rammensee HG, Steinle A . Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol 2002; 169: 4098–4102.
Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O’Reilly RJ, Dupont B et al. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol 2003; 171: 6891–6899.
Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR . Soluble MICB in malignant diseases: analysis of diagnostic significance and correlation with soluble MICA. Cancer Immunol Immunother 2006; 55: 1584–1589.
Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR . Soluble MICA in malignant diseases. Int J Cancer 2006; 118: 684–687.
Ganem NJ, Pellman D . Limiting the proliferation of polyploid cells. Cell 2007; 131: 437–440.
Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M et al. An immunosurveillance mechanism controls cancer cell ploidy. Science 2012; 337: 1678–1684.
Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005; 123: 321–334.
Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ . Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res 2011; 71: 4809–4820.
Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G . The anticancer immune response: indispensable for therapeutic success? J Clin Investig 2008; 118: 1991–2001.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13: 54–61.
Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15: 1170–1178.
Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med 2011; 208: 491–503.
Fregni G, Perier A, Pittari G, Jacobelli S, Sastre X, Gervois N et al. Unique functional status of natural killer cells in metastatic stage IV melanoma patients and its modulation by chemotherapy. Clin Cancer Res 2011; 17: 2628–2637.
Hsu AK, Quach H, Tai T, Prince HM, Harrison SJ, Trapani JA et al. The immunostimulatory effect of lenalidomide on NK-cell function is profoundly inhibited by concurrent dexamethasone therapy. Blood 2011; 117: 1605–1613.
Acknowledgements
CJC is supported by a Leukaemia Foundation of Australia PhD scholarship. MJS and LM are supported by a NHMRC Australia Fellowship, Program, and Project Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Piacentini
Rights and permissions
About this article
Cite this article
Chan, C., Smyth, M. & Martinet, L. Molecular mechanisms of natural killer cell activation in response to cellular stress. Cell Death Differ 21, 5–14 (2014). https://doi.org/10.1038/cdd.2013.26
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2013.26
Keywords
This article is cited by
-
Immunotherapies for Pediatric Solid Tumors: A Targeted Update
Pediatric Drugs (2022)
-
Small-molecule HDAC and Akt inhibitors suppress tumor growth and enhance immunotherapy in multiple myeloma
Journal of Experimental & Clinical Cancer Research (2021)
-
Overcoming the UCB HSCs –Derived NK cells Dysfunction through Harnessing RAS/MAPK, IGF-1R and TGF-β Signaling Pathways
Cancer Cell International (2021)
-
Rac1/ROCK-driven membrane dynamics promote natural killer cell cytotoxicity via granzyme-induced necroptosis
BMC Biology (2021)
-
A comprehensive analysis of TDO2 expression in immune cells and characterization of immune cell phenotype in TDO2 knockout mice
Transgenic Research (2021)