Abstract
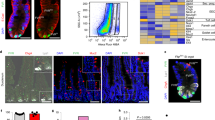
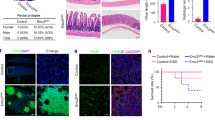
Paneth cells (PCs), a secretory population located at the base of the intestinal crypt, support the intestinal stem cells (ISC) with growth factors and participate in innate immunity by releasing antimicrobial peptides, including lysozyme and defensins. PC dysfunction is associated with disorders such as Crohn’s disease and necrotizing enterocolitis, but the specific pathways regulating PC development and function are not fully understood. Here we tested the role of the neuregulin receptor ErbB3 in control of PC differentiation and the ISC niche. Intestinal epithelial ErbB3 knockout caused precocious appearance of PCs as early as postnatal day 7, and substantially increased the number of mature PCs in adult mouse ileum. ErbB3 loss had no effect on other secretory lineages, but increased expression of the ISC marker Lgr5. ErbB3-null intestines had elevated levels of the Atoh1 transcription factor, which is required for secretory fate determination, while Atoh1+ cells had reduced ErbB3, suggesting reciprocal negative regulation. ErbB3-null intestinal progenitor cells showed reduced activation of the PI3K–Akt and ERK MAPK pathways. Inhibiting these pathways in HT29 cells increased levels of ATOH1 and the PC marker LYZ. Conversely, ErbB3 activation suppressed LYZ and ATOH1 in a PI3K-dependent manner. Expansion of the PC compartment in ErbB3-null intestines was accompanied with elevated ER stress and inflammation markers, raising the possibility that negative regulation of PCs by ErbB3 is necessary to maintain homeostasis. Taken together, our data suggest that ErbB3 restricts PC numbers through PI3K-mediated suppression of Atoh1 levels leading to inhibition of PC differentiation, with important implications for regulation of the ISC niche.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cheng H . Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. IV. Paneth cells. Am J Anat 1974; 141: 521–535.
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449: 1003–1007.
Peeters T, Vantrappen G . The Paneth cell: a source of intestinal lysozyme. Gut 1975; 16: 553–558.
Ouellette AJ, Greco RM, James M, Frederick D, Naftilan J, Fallon JT . Developmental regulation of cryptdin, a corticostatin defensin precursor messenger-RNA in mouse small inestinal crypt epithelium. J Cell Biol 1989; 108: 1687–1695.
Jones DE, Bevins CL . Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem 1992; 267: 23216–23225.
Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011; 469: 415–418.
Lewin K . The Paneth cell in disease. Gut 1969; 10: 804–811.
Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE et al. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA 2005; 102: 18129–18134.
Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008; 456: 259–U262.
McElroy SJ, Underwood MA, Sherman MP . Paneth cells and necrotizing enterocolitis: a novel hypothesis for disease pathogenesis. Neonatology 2013; 103: 10–20.
Adolph TE, Tomczak MF, Niederreiter L, Ko HJ, Bock J, Martinez-Naves E et al. Paneth cells as a site of origin for intestinal inflammation. Nature 2013; 503: 272–276.
Andreu P, Peignon G, Slomianny C, Taketo MM, Colnot S, Robine S et al. A genetic study of the role of the Wnt/beta-catenin signalling in Paneth cell differentiation. Dev Biol 2008; 324: 288–296.
VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 2012; 139: 488–497.
Heuberger J, Kosel F, Qi JJ, Grossmann KS, Rajewsky K, Birchmeier W . Shp2/MAPK signaling controls goblet/paneth cell fate decisions in the intestine. Proc Natl Acad Sci USA 2014; 111: 3472–3477.
Jones JT, Akita RW, Sliwkowski MX . Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett 1999; 447: 227–231.
Prigent SA, Gullick WJ . Identification of c-erbB-3 binding sites for phosphatidylinositol 3'-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J 1994; 13: 2831–2841.
Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL 3rd . Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci U S A 1994; 91: 8132–8136.
Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA . ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA 2010; 107: 7692–7697.
Lee D, Yu M, Lee E, Kim H, Yang Y, Kim K et al. Tumor-specific apoptosis caused by deletion of the ERBB3 pseudo-kinase in mouse intestinal epithelium. J Clin Invest 2009; 119: 2702–2713.
Zhang Y, Dube PE, Washington MK, Yan F, Polk DB . ErbB2 and ErbB3 regulate recovery from dextran sulfate sodium-induced colitis by promoting mouse colon epithelial cell survival. Lab Invest 2012; 92: 437–450.
McElroy SJ, Castle SL, Bernard JK, Almohazey D, Hunter CJ, Bell BA et al. The ErbB4 ligand Neuregulin-4 protects against experimental necrotizing enterocolitis. Am J Pathol 2014; 184: 2768–2778.
VanDussen KL, Liu TC, Li DL, Towfic F, Modiano N, Winter R et al. Genetic variants synthesize to produce Paneth cell phenotypes that define subtypes of Crohn's disease. Gastroenterology 2014; 146: 200–209.
Bry L, Falk P, Huttner K, Ouellette A, Midtvedt T, Gordon JI . Paneth cell-differentaition in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci USA 1994; 91: 10335–10339.
Troughton WD, Trier JS . Paneth and goblet cell renewal in mouse duodenal crypts. J Cell Biol 1969; 41: 251–68.
Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY . Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 2001; 294: 2155–2158.
Shroyer NF, Helmrath MA, Wang VYC, Antalffy B, Henning SJ, Zoghbi HY . Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology 2007; 132: 2478–2488.
Bernard JK, McCann SP, Bhardwaj V, Washington MK, Frey MR . Neuregulin-4 is a survival factor for colon epithelial cells both in culture and in vivo. J Biol Chem 2012; 287: 39850–39858.
Tanaka M, Saito H, Kusumi T, Fukuda S, Shimoyama T, Sasaki Y et al. Spatial distribution and histogenesis of colorectal Paneth cell metaplasia in idiopathic inflammatory bowel disease. J Gastroenterol Hepatol 2001; 16: 1353–1359.
Simmonds N, Furman M, Karanika E, Phillips A, Bates AW . Paneth cell metaplasia in newly diagnosed inflammatory bowel disease in children. BMC Gastroenterol 2014; 14: 93.
Huet C, Sahuquillomerino C, Coudrier E, Louvard D . Absorptive and mucus-secreting subclones isolated from a multipotent intestinal-cell line (HT29) provide new models for cell polarity and terminal differentiation. J Cell Biol 1987; 105: 345–357.
Zhao F, Edwards R, Dizon D, Mastroianni JR, Geyfman M, Ouellette AJ et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev Biol 2010; 338: 270–279.
Frey MR, Edelblum KL, Mullane MT, Liang D, Polk DB . The ErbB4 Growth Factor Receptor Is Required for Colon Epithelial Cell Survival in the Presence of TNF. Gastroenterology 2009; 136: 217–226.
Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med 2014; 20: 1436–1443.
Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J 1996; 15: 2452–2467.
Berger MB, Mendrola JM, Lemmon MA . ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett 2004; 569: 332–336.
Telesco SE, Shih AJ, Jia F, Radhakrishnan R . A multiscale modeling approach to investigate molecular mechanisms of pseudokinase activation and drug resistance in the HER3/ErbB3 receptor tyrosine kinase signaling network. Mol Biosyst 2011; 7: 2066–2080.
Steinkamp MP, Low-Nam ST, Yang S, Lidke KA, Lidke DS, Wilson BS . erbB3 is an active tyrosine kinase capable of homo- and heterointeractions. Mol Cell Biol 2014; 34: 965–977.
Hellyer NJ, Cheng K, Koland JG . ErbB3 (HER3) interaction with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem J 1998; 333 (Pt 3): 757–763.
Laederich MB, Funes-Duran M, Yen L, Ingalla E, Wu X, Carraway KL 3rd et al. The leucine-rich repeat protein LRIG1 is a negative regulator of ErbB family receptor tyrosine kinases. J Biol Chem 2004; 279: 47050–47056.
Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol 2012; 14: 401–408.
Grun D, Lyubimova A, Kester L, Wiebrands K, Basak O, Sasaki N et al. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 2015; 525: 251–255.
Feng Y, Teitelbaum DH . Epidermal growth factor/TNF-α transactivation modulates epithelial cell proliferation and apoptosis in a mouse model of parenteral nutrition. Am J Physiol Gastrointest Liver Physiol 2012; 302: G236–G249.
Li X, Madison BB, Zacharias W, Kolterud A, States D, Gumucio DL . Deconvoluting the intestine: molecular evidence for a major role of the mesenchyme in the modulation of signaling cross talk. Physiol Genomics 2007; 29: 290–301.
Mitsui K, Yonezawa M, Tatsuguchi A, Shinji S, Gudis K, Tanaka S et al. Localization of phosphorylated ErbB1-4 and heregulin in colorectal cancer. BMC Cancer 2014; 14: 863.
Al Alam D, Danopoulos S, Schall K, Sala FG, Almohazey D, Fernandez GE et al. Fibroblast growth factor 10 alters the balance between goblet and Paneth cells in the adult mouse small intestine. Am J Physiol Gastrointest Liver Physiol 2015; 308: G678–G690.
Aliaga JC, Deschenes C, Beaulieu JF, Calvo EL, Rivard N . Requirement of the MAP kinase cascade for cell cycle progression and differentiation of human intestinal cells. Am J Physiol Gastrointest Liver Physiol 1999; 277 (3 Pt 1): G631–G641.
Lemieux E, Boucher MJ, Mongrain S, Boudreau F, Asselin C, Rivard N . Constitutive activation of the MEK/ERK pathway inhibits intestinal epithelial cell differentiation. Am J Physiol Gastrointest Liver Physiol 2011; 301: G719–G730.
Robles AI, Traverso G, Zhang M, Roberts NJ, Khan MA, Joseph C et al. Whole-exome sequencing analyses of inflammatory bowel disease-associated colorectal cancers. Gastroenterology 2016; 150: 931–943.
Long W, Wagner KU, Lloyd KC, Binart N, Shillingford JM, Hennighausen L et al. Impaired differentiation and lactational failure of Erbb4-deficient mammary glands identify ERBB4 as an obligate mediator of STAT5. Development 2003; 130: 5257–5268.
Rose MF, Ren J, Ahmad KA, Chao HT, Klisch TJ, Flora A et al. Math1 is essential for the development of hindbrain neurons critical for perinatal breathing. Neuron 2009; 64: 341–354.
Williams JM, Duckworth CA, Watson AJM, Frey MR, Miguel JC, Burkitt MD et al. A mouse model of pathological small intestinal epithelial cell apoptosis and shedding induced by systemic administration of lipopolysaccharide. Dis Model Mech 2013; 6: 1388–1399.
Perret V, Lev R, Pigman W . Simple method for the preparation of single cell suspensions from normal and tumorous rat colonic mucosa. Gut 1977; 18: 382–385.
Simmons AJ, Banerjee A, McKinley ET, Scurrah CR, Herring CA, Gewin LS et al. Cytometry-based single-cell analysis of intact epithelial signaling reveals MAPK activation divergent from TNF-alpha-induced apoptosis in vivo. Mol Syst Biol 2015; 11: 835.
Mahe MM, Aihara E, Schumacher MA, Zavros Y, Montrose MH, Helmrath MA et al. Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol 2013; 3: 217–240.
Whitehead RH, Robinson PS . Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice. Am J Physiol Gastrointest Liver Physiol 2009; 296: G455–G460.
Klisch TJ, Xi Y, Flora A, Wang L, Li W, Zoghbi HY . In vivo Atoh1 targetome reveals how a proneural transcription factor regulates cerebellar development. Proc Natl Acad Sci USA 2011; 108: 3288–3293.
Lo YH, Chung E, Li Z, Wan YW, Mahe MM, Chen MS et al. Transcriptional regulation by ATOH1 and its target SPDEF in the intestine. Cell Mol Gastroenterol Hepatol 2017; 3: 51–71.
Acknowledgements
We thank the Dixon Cellular Imaging Core at The Saban Research Institute for expert assistance. This work was supported by National Institutes of Health awards R01DK095004 (to MRF), R01DK103831, P30CA068485, P50CA095103, P30DK058404 (to KSL), R01CA142826, R01DK092306 (to NFS), K01DK100485 (to KEH), by a Senior Research Award from the Crohn’s and Colitis Foundation of America (to MRF), by a Research Scholar Grant from the American Cancer Society (to MRF), and by a fellowship from the Institute for Research and Medical Consultations at the University of Dammam (DA).
Author contributions
DA, Y-HL, MAS, KEH, KSL, NFS and MRF conceived and designed the experiments. DA, Y-HL, CVV, AJS, JJH, EBB, MAS, KSL, NFS and MRF performed the experiments and analyzed the data. DA, Y-HL, NFS and MRF wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by P Bouillet
Rights and permissions
About this article
Cite this article
Almohazey, D., Lo, YH., Vossler, C. et al. The ErbB3 receptor tyrosine kinase negatively regulates Paneth cells by PI3K-dependent suppression of Atoh1. Cell Death Differ 24, 855–865 (2017). https://doi.org/10.1038/cdd.2017.27
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2017.27
This article is cited by
-
Afatinib amplifies cAMP-induced fluid secretion in a mouse mini-gut model via TMEM16A-mediated fluid secretion and secretory cell differentiation
Scientific Reports (2025)
-
Supplementation with the Probiotic Strains Bifidobacterium longum and Lactiplantibacillus rhamnosus Alleviates Glucose Intolerance by Restoring the IL-22 Response and Pancreatic Beta Cell Dysfunction in Type 2 Diabetic Mice
Probiotics and Antimicrobial Proteins (2025)
-
Neuregulin-1 signaling regulates cytokines and chemokines expression and secretion in granulosa cell
Journal of Ovarian Research (2022)
-
Sprouty2 limits intestinal tuft and goblet cell numbers through GSK3β-mediated restriction of epithelial IL-33
Nature Communications (2021)
-
Plasticity of Paneth cells and their ability to regulate intestinal stem cells
Stem Cell Research & Therapy (2020)