Abstract
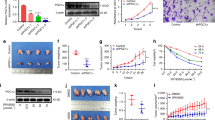
Peroxisome proliferator-activated receptor gamma (PPARγ) coactivator-1 alpha (PGC-1α) coactivates multiple transcription factors and regulates several metabolic processes. The current study investigated the role of PGC-1α in the induction of apoptosis in human epithelial ovarian cancer cells. The PGC-1α mRNA level between human ovaries and human ovarian epithelial tumors was examined by quantitative RT-PCR. Less PGC-1α expression was found in the surface epithelium of malignant tumors compared with normal ovaries. Overexpression of PGC-1α in human epithelial ovarian cancer cell line Ho-8910 induced cell apoptosis through the coordinated regulation of Bcl-2 and Bax expression. Microarray analyses confirmed that PGC-1α dramatically affected the apoptosis-related genes in Ho-8910 cells. Mitochondrial functional assay showed that the induction of apoptosis was through the terminal stage by the release of cytochrome c. Furthermore, PGC-1α-induced apoptosis was partially, but not completely, blocked by PPARγ antagonist (GW9662), and suppression of PPARγ expression by siRNA also inhibited PGC-1α-induced apoptosis in Ho-8910 cells. These data suggested that PGC-1α exerted its effect through a PPARγ-dependent pathway. Our findings indicated that PGC-1α was involved in the apoptotic signal transduction pathways and downregulation of PGC-1α may be a key point in promoting epithelial ovarian cancer growth and progression.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jemal A, Thomas A, Murray T, Thun M . Cancer statistics. CA Cancer J Clin 2002; 52:23–47.
Wooster R, Weber BL . Breast and ovarian cancer. N Engl J Med 2003; 348:2339–2347.
Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348:203–213.
Puigserver P, Spiegelman BM . Peroxisome proliferator-activated receptor-coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev 2003; 24:78–90.
Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999; 98:115–124.
Larrouy D, Vidal H, Andreelli F, Laville M, Langin D . Cloning and mRNA tissue distribution of human PPARgamma coactivator-1. Int J Obes Relat Metab Disord 1999; 23:1327–1332.
Esterbauer H, Oberkofler H, Krempler F, Patsch W . Human peroxisome proliferator activated receptor gamma coactivator 1 (PPARGC1) gene: cDNA sequence, genomic organization, chromosomal localization, and tissue expression. Genomics 1999; 62:98–102.
Puigserver P, Wu Z, Park CW, Graves R, Wright W, Spiegelman BM . A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998; 92:829–839.
Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 2002; 418:797–801.
Yoon JC, Puigserver P, Chen G, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 2001; 413:131–138.
Yoon JC, Xu G, Deeney JT, et al. Suppression of β cell energy metabolism and insulin release by PGC-1α. Dev Cell 2003; 5:73–83.
Jiang WG, Douglas-Jones A, Mansel RE . Expression of peroxisome-proliferator activated receptor-gamma (PPARgamma) and the PPARgamma co-activator, PGC-1, in human breast cancer correlates with clinical outcomes. Int J Cancer 2003; 106:752–757.
Watkins G, Douglas-Jones A, Mansel RE, Jiang WG . The localisation and reduction of nuclear staining of PPARgamma and PGC-1 in human breast cancer. Oncol Rep 2004; 12:483–488.
Feilchenfeldt J, Brundler MA, Soravia C, Totsch M, Meier CA . Peroxisome proliferator-activated receptors (PPARs) and associated transcription factors in colon cancer: reduced expression of PPARgamma-coactivator 1 (PGC-1). Cancer Lett 2004; 203:25–33.
Castillo G, Brun RP, Rosenfield JK, et al. An adipogenic cofactor bound by the differentiation domain of PPAR gamma. EMBO J 1999; 18:3676–3687.
Puigserver P, Adelmant C, Wu ZD, et al. Activation of PPAR gamma coactivator-1 through transcription factor docking. Science 1999; 286:1368–1371.
Lefebvre AM, Chen I, Desreumaux P, et al. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med 1998; 4:1053–1057.
Saez E, Tontonoz P, Nelson MC, et al. Activators of the nuclear receptor PPARγ enhance colon polyp formation. Nat Med 1998; 4:1058–1061.
Saez E, Rosenfeld J, Livolsi A, et al. PPARγ signaling exacerbates mammary gland tumor development. Genes Dev 2004; 18:528–540.
Koeffler HP . Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res 2003; 9:1–9.
Park BH, Breyer B, He TC . Peroxisome proliferator-activated receptors: roles in tumorigenesis and chemoprevention in human cancer. Curr Opin Oncol 2001; 13:78–83.
Rumi MA, Ishihara S, Kazumori H, Kadowaki Y, Kinoshita Y . Can PPAR gamma ligands be used in cancer therapy? Curr Med Chem Anticancer Agents 2004; 4:465–477.
Zhang GY, Ahmed N, Riley C, et al. Enhanced expression of peroxisome proliferator-activated receptor gamma in epithelial ovarian carcinoma. Br J Cancer 2005; 92:113–119.
No authors listed. International Federation of Gynecology, and Obstetrics: changes in definitions of clinical staging for carcinoma of the cervix and ovary. Am J Obstet Gynecol 1987; 156:263–264.
Mou Z, Xu SH, Zhang YY . Constitution of human ovarian cancer cell line HO8910 and its biological characteristic. Zhong Hua Fu Chan Ke Za Zhi 1994; 29:162–164.
Ma W, Yu H, Wang Q, Bao J, Yan J, Jin H . In vitro biological activities of transmembrane superantigen staphylococcal enterotoxin A fusion protein. Cancer Immunol Immunother 2004; 53:118–124.
Zhang J, Wang X, Tu C, et al. Monofunctional platinum complexes showing potent cytotoxicity against human liver carcinoma cell line BEL-7402. J Med Chem 2003; 46:3502–3507.
Miyake S, Makimura M, Kanegae Y, et al. Efficient generation of recombinant adenoviruses using adenovirus DNA-termal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci USA 1996; 93:1320–1324.
Nicoletti I, Migliorati G, Pagliacci M, Grignani F, Riccardi C . A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flowcytometry. J Immunol Methods 1991; 139:271–279.
Yang CC, Lin HP, Chen CS, Yang YT, Tseng PH, Rangnekar VM . Bcl-xL mediates a survival mechanism independent of the phosphoinositide 3-kinase/Akt pathway in prostate cancer cells. J Biol Chem 2003; 278:25872–25878.
Yang YH, Dudoit S, Luu P, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 2002; 30:e15.
Choi KC, Auersperg N . The ovarian surface epithelium: simple source of a complex disease. Minerva Ginecol 2003; 55:297–314.
Zhang GW, Ahmed N, Riley C, et al. Enhanced expression of peroxisome proliferator-activated receptor gamma in epithelial ovarian carcinoma. Br J Cancer 2005; 92:113–119.
Hu ZY, Deng XG . Effects of progesterone to human ovarian cancer cell line HO8910's proliferation and apoptosis. Zhong Hua Fu Chang Ke Za Zhi 2000; 35:423–426.
Shen ZN, Nishida K, Doi H, et al. Suppression of chondrosarcoma cells by 15-deoxy-Delta 12,14-prostaglandin J2 is associated with altered expression of Bax/Bcl-xL and p21. Biochem Biophys Res Commun 2005; 328:375–382.
Cory S, Adams JM . The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002; 2:647–656.
Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN . Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol 1993; 4:327–332.
Cai J, Yang J, Jones DP . Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta 1998; 1366:139–149.
Tsujimoto Y . Cell death regulation by the bcl-2 protein family in the mitochondria. J Cell Physiol 2003; 195:158–167.
Shiau CW, Yang CC, Kulp SK, et al. Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARgamma. Cancer Res 2005; 65:1561–1569.
Kang HY, Lee JY, Lee JS, Choi YM . Peroxisome proliferator-activated receptors-gamma activator, ciglitazone, inhibits human melanocyte growth through induction of apoptosis. Arch Dermatol Res 2006; 297:472–476.
Li MY, Deng H, Zhao JM, Dai D, Tan XY . PPARgamma pathway activation results in apoptosis and COX-2 inhibition in HepG2 cells. World J Gastroenterol 2003; 9:1220–1226.
Bogazzi F, Russo D, Locci MT, et al. Apoptosis is reduced in the colonic mucosa of patients with acromegaly. Clin Endocrinol (Oxf) 2005; 63:683–688.
Acknowledgements
We thank Rongxi Sun from The first Affiliated Hospital of Nanjing Medical University for kindly providing the human ovarian specimens. This work was supported by grants from the National Natural Science Foundation of China (No. 30225037, 30400538, 30471991, 30570731), the 973 Program of China (No. 2006CB503909, 2004CB518603), the “111” Project, and the Natural Science Foundation of Jiangsu Province (No. BK2004082, BK2006714).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Y., Ba, Y., Liu, C. et al. PGC-1α induces apoptosis in human epithelial ovarian cancer cells through a PPARγ-dependent pathway. Cell Res 17, 363–373 (2007). https://doi.org/10.1038/cr.2007.11
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2007.11
Keywords
This article is cited by
-
Ginsenoside-Rg2 exerts anti-cancer effects through ROS-mediated AMPK activation associated mitochondrial damage and oxidation in MCF-7 cells
Archives of Pharmacal Research (2021)
-
Amplification of Mitochondrial Activity in the Healing Response Following Rotator Cuff Tendon Injury
Scientific Reports (2018)
-
Neuromuscular Junction Morphology and Gene Dysregulation in the Wobbler Model of Spinal Neurodegeneration
Journal of Molecular Neuroscience (2018)
-
PGC-1α attenuates hydrogen peroxide-induced apoptotic cell death by upregulating Nrf-2 via GSK3β inactivation mediated by activated p38 in HK-2 Cells
Scientific Reports (2017)
-
Oestrogen Receptor-α binds the FOXP3 promoter and modulates regulatory T-cell function in human cervical cancer
Scientific Reports (2017)