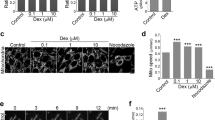
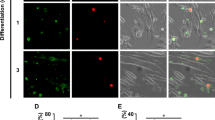
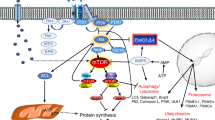
Abstract
The growing interest in skeletal muscle regeneration is associated with the opening of new therapeutic strategies for muscle injury after trauma, as well as several muscular degenerative pathologies, including dystrophies, muscular atrophy, and cachexia. Studies focused on the ability of extracellular factors to promote myogenesis are therefore highly promising. We now report that an adipocyte-derived factor, globular adiponectin (gAd), is able to induce muscle gene expression and cell differentiation. gAd, besides its well-known ability to regulate several metabolic functions in muscle, including glucose uptake and consumption and fatty acid catabolism, is able to block cell cycle entry of myoblasts, to induce the expression of specific skeletal muscle markers such as myosin heavy chain or caveolin-3, as well as to provoke cell fusion into multinucleated syncytia and, finally, muscle fibre formation. gAd exerts its pro-differentiative activity through redox-dependent activation of p38, Akt and 5′-AMP-activated protein kinase pathways. Interestingly, differentiating myoblasts are autocrine for adiponectin, and the mimicking of pro-inflammatory settings or exposure to oxidative stress strongly increases the production of the hormone from differentiating cells. These data suggest a novel function of adiponectin, directly coordinating the myogenic differentiation program and serving an autocrine function during skeletal myogenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- gAd:
-
(globular adiponectin)
- DM:
-
(differentiation medium)
- mMHC:
-
(muscle myosin heavy chain)
- HOS:
-
(horse serum)
- ROS:
-
(reactive oxygen species)
References
Pajvani UB, Du X, Combs TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem 2003; 278:9073–9085.
Berg AH, Combs TP, Scherer PE . ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab 2002; 13:84–89.
Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 2001; 98:2005–2010.
Kadowaki T, Yamauchi T . Adiponectin and adiponectin receptors. Endocr Rev 2005; 26:439–451.
Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7:941–946.
Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003; 423:762–769.
Yamauchi T, Nio Y, Maki T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 2007; 13:332–339.
Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L . Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest 2001; 108:1875–1881.
Fiaschi T, Buricchi F, Cozzi G, et al. Redox-dependent and ligand-independent trans-activation of insulin receptor by globular adiponectin. Hepatology 2007; 46:130–139.
Yamauchi T, Kamon J, Minokoshi, Y et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002; 8:1288–1295.
Mao X, Kikani CK, Riojas RA, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 2006; 8:516–523.
Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K . Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006; 116:1784–1792.
Blau HM, Pavlath GK, Hardeman EC, et al. Plasticity of the differentiated state. Science 1985; 230:758–766.
Charge SB, Rudnicki MA . Cellular and molecular regulation of muscle regeneration. Physiol Rev 2004; 84:209–238.
Molkentin JD, Olson EN . Defining the regulatory networks for muscle development. Curr Opin Genet Dev 1996; 6:445–453.
Bach LA, Salemi R, Leeding KS . Roles of insulin-like growth factor (IGF) receptors and IGF-binding proteins in IGF-II-induced proliferation and differentiation of L6A1 rat myoblasts. Endocrinology 1995; 136:5061–5069.
Ewton DZ, Roof SL, Magri KA, McWade FJ, Florini JR . IGF-II is more active than IGF-I in stimulating L6A1 myogenesis: greater mitogenic actions of IGF-I delay differentiation. J Cell Physiol 1994; 161:277–284.
Florini JR, Ewton DZ, Magri KA, Mangiacapra FJ . IGFs and muscle differentiation. Adv Exp Med Biol 1993; 343:319–326.
Conejo R, Valverde AM, Benito M, Lorenzo M . Insulin produces myogenesis in C2C12 myoblasts by induction of NF-kappaB and downregulation of AP-1 activities. J Cell Physiol 2001; 186:82–94.
Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR . The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 1997; 272:6653–6662.
Donati C, Meacci E, Nuti F, Becciolini L, Farnararo M, Bruni P . Sphingosine 1-phosphate regulates myogenic differentiation: a major role for S1P2 receptor. FASEB J 2005; 19:449–451.
Filigheddu N, Gnocchi VF, Coscia M, et al. Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol Biol Cell 2007; 18:986–994.
Li Y, Jiang B, Ensign WY, Vogt PK, Han J . Myogenic differentiation requires signalling through both phosphatidylinositol 3-kinase and p38 MAP kinase. Cell Signal 2000; 12:751–757.
Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7:941–946.
Furtado LM, Somwar R, Sweeney G, Niu W, Klip A . Activation of the glucose transporter GLUT4 by insulin. Biochem Cell Biol 2002; 80:569–578.
Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM . Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology 2004; 145:5589–5597.
Buckingham M . Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev 2006; 16:525–532.
Armand AS, Laziz I, Chanoine C . FGF6 in myogenesis. Biochim Biophys Acta 2006; 1763:773–778.
Floss T, Arnold HH, Braun T . A role for FGF-6 in skeletal muscle regeneration. Genes Dev 1997; 11:2040–2051.
Andres V, Walsh K . Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol 1996; 132:657–666.
Heron-Milhavet L, Mamaeva D, Rochat A, Lamb NJ, Fernandez A . Akt2 is implicated in skeletal muscle differentiation and specifically binds Prohibitin2/REA. J Cell Physiol 2008; 214:158–165.
Wu Z, Woodring PJ, Bhakta KS, et al. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol 2000; 20:3951–3964.
Long YC, Zierath JR . AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest 2006; 116:1776–1783.
Waki H, Yamauchi T, Kamon J, et al. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology 2005; 146:790–796.
Pierce AP, de Waal E, McManus LM, Shireman PK, Chaudhuri AR . Oxidation and structural perturbation of redox-sensitive enzymes in injured skeletal muscle. Free Radic Biol Med 2007; 43:1584–1593.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25:402–408.
Acknowledgements
This work was supported by the Tuscany Region Studies on Rosiglitazone (TRESOR), the Italian Association for Cancer Research (AIRC), the Consorzio Interuniversitario Biotecnologie and the Cassa di Risparmio di Firenze.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fiaschi, T., Cirelli, D., Comito, G. et al. Globular adiponectin induces differentiation and fusion of skeletal muscle cells. Cell Res 19, 584–597 (2009). https://doi.org/10.1038/cr.2009.39
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2009.39
Keywords
This article is cited by
-
Recent advances and future avenues in understanding the role of adipose tissue cross talk in mediating skeletal muscle mass and function with ageing
GeroScience (2021)
-
Role of adiponectin in the metabolism of skeletal muscles in collagen VI–related myopathies
Journal of Molecular Medicine (2019)
-
Asymmetric expression of H19 and ADIPOQ in concave/convex paravertebral muscles is associated with severe adolescent idiopathic scoliosis
Molecular Medicine (2018)
-
Skeletal muscle secretome in Duchenne muscular dystrophy: a pivotal anti-inflammatory role of adiponectin
Cellular and Molecular Life Sciences (2017)
-
Involvement of adiponectin in the pathogenesis of dystrophinopathy
Skeletal Muscle (2015)