Abstract
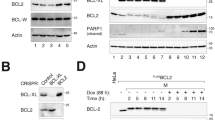
BCL2 is best known as a multifunctional anti-apoptotic protein. However, little is known about its role in cell-adhesive and motility events. Here, we show that BCL2 may play a role in the regulation of cell adhesion, spreading, and motility. When BCL2 was overexpressed in cultured murine and human cell lines, cell spreading, adhesion, and motility were impaired. Consistent with these results, the loss of Bcl2 resulted in higher motility observed in Bcl2-null mouse embryonic fibroblast (MEF) cells compared to wild type. The mechanism of BCL2 regulation of cell adhesion and motility may involve formation of a complex containing BCL2, actin, and gelsolin, which appears to functionally decrease the severing activity of gelsolin. We have observed that the lysate from MCF-7 and NIH3T3 cells that overexpressed BCL2 enhanced actin polymerization in cell-free in vitro assays. Confocal immunofluorescent localization of BCL2 and F-actin during spreading consistently showed that increased expression of BCL2 resulted in increased F-actin polymerization. Thus, the formation of BCL2 and gelsolin complexes (which possibly contain other proteins) appears to play a critical role in the regulation of cell adhesion and migration. Given the established correlation of cell motility with cancer metastasis, this result may explain why the expression of BCL2 in some tumor cell types reduces the potential for metastasis and is associated with improved patient prognosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- BCL2 :
-
(human gene)
- Bcl2 :
-
(mouse gene)
- BCL2:
-
(human and mouse protein)
References
Reed JC . Double identity for proteins of the Bcl-2 family. Nature 1997; 387:773–776.
Strasser A, Harris AW, Cory S . E mu-bcl-2 transgene facilitates spontaneous transformation of early pre-B and immunoglobulin-secreting cells but not T cells. Oncogene 1993; 8:1–9.
Kumar P, Ning Y, Polverini PJ . Endothelial cells expressing Bcl-2 promotes tumor metastasis by enhancing tumor angiogenesis, blood vessel leakiness and tumor invasion. Lab Invest 2008; 88:740–749.
Yilmaz A, Savas I, Dizbay Sak S, et al. Distribution of Bcl-2 gene expression and its prognostic value in non-small cell lung cancer. Tuberk Toraks 2005; 53:323–329.
Itoi T, Yamana K, Bilim V, Takahashi K, Tomita F . Impact of frequent Bcl-2 expression on better prognosis in renal cell carcinoma patients. Br J Cancer 2004; 90:200–205.
Leahy DT, Mulcahy HE, O'Donoghue DP, Parfrey NA . bcl-2 protein expression is associated with better prognosis in colorectal cancer. Histopathology 1999; 35:360–367.
Divito KA, Berger AJ, Camp RL, et al. Automated quantitative analysis of tissue microarrays reveals an association between high Bcl-2 expression and improved outcome in melanoma. Cancer Res 2004; 64:8773–8777.
Neri A, Marrelli D, Roviello F, et al. Bcl-2 expression correlates with lymphovascular invasion and long-term prognosis in breast cancer. Breast Cancer Res Treat 2006; 99:77–83.
Rathmell JC, Thompson CB . Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell 2002; 109 Suppl:S97–S107.
Akiyama SK, Olden K, Yamada KM . Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev 1995; 14:173–189.
Bokel C, Brown NH . Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev Cell 2002; 3:311–321.
Kedrin D, van Rheenen J, Hernandez L, Condeelis J, Segall JE . Cell motility and cytoskeletal regulation in invasion and metastasis. J Mammary Gland Biol Neoplasia 2007; 12:143–152.
Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science 2003; 302:1704–1709.
Webb DJ, Brown CM, Horwitz AF . Illuminating adhesion complexes in migrating cells: moving toward a bright future. Curr Opin Cell Biol 2003; 15:614–620.
Disanza A, Steffen A, Hertzog M, et al. Actin polymerization machinery: the finish line of signaling networks, the starting point of cellular movement. Cell Mol Life Sci 2005; 62:955–970.
Kassis J, Lauffenburger DA, Turner T, Wells A . Tumor invasion as dysregulated cell motility. Semin Cancer Biol 2001; 11:105–117.
Pantaloni D, Le Clainche C, Carlier MF . Mechanism of actin-based motility. Science 2001; 292:1502–1506.
Pollard TD . The cytoskeleton, cellular motility and the reductionist agenda. Nature 2003; 422:741–745.
Small JV, Stradal T, Vignal E, Rottner K . The lamellipodium: where motility begins. Trends Cell Biol 2002; 12:112–120.
Silacci P, Mazzolai L, Gauci C, et al. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci 2004; 61:2614–2623.
Linder S, Aepfelbacher M . Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol 2003; 13:376–385.
De Corte V, Bruyneel E, Boucherie C, et al. Gelsolin-induced epithelial cell invasion is dependent on Ras-Rac signaling. EMBO J 2002; 21:6781–6790.
Larson L, Arnaudeau S, Gibson B, et al. Gelsolin mediates calcium-dependent disassembly of Listeria actin tails. Proc Natl Acad Sci USA 2005; 102:1921–1926.
Azuma T, Witke W, Stossel TP, Hartwig JH, Kwiatkowski DJ . Gelsolin is a downstream effector of rac for fibroblast motility. EMBO J 1998; 17:1362–1370.
Cunningham CC, Stossel TP, Kwiatkowski DJ . Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science 1991; 251:1233–1236.
Arora PD, McCulloch CA . Dependence of fibroblast migration on actin severing activity of gelsolin. J Biol Chem 1996; 271:20516–20523.
Akiyama SK . Functional analysis of cell adhesion: quantitation of cell-matrix attachment. Methods Cell Biol 2002; 69:281–296.
Gildea JJ, Harding MA, Gulding KM, Theodorescu D . Transmembrane motility assay of transiently transfected cells by fluorescent cell counting and luciferase measurement. Biotechniques 2000; 29:81–86.
Reinhart-King CA, Dembo M, Hammer DA . The dynamics and mechanics of endothelial cell spreading. Biophys J 2005; 89:676–689.
Apostolova MD, Christova T, Templeton DM . Involvement of gelsolin in cadmium-induced disruption of the mesangial cell cytoskeleton. Toxicol Sci 2006; 89:465–474.
Li S, Guan JL, Chien S . Biochemistry and biomechanics of cell motility. Annu Rev Biomed Eng 2005; 7:105–150.
Zhang W, Pantschenko AG, McCarthy MB, Gronowicz G . Bone-targeted overexpression of Bcl-2 increases osteoblast adhesion and differentiation and inhibits mineralization in vitro. Calcif Tissue Int 2007; 80:111–122.
Sheibani N, Scheef EA, Dimaio TA, et al. Bcl-2 expression modulates cell adhesion and migration promoting branching of ureteric bud cells. J Cell Physiol 2007; 210:616–625.
Lambrechts A, Van Troys M, Ampe C . The actin cytoskeleton in normal and pathological cell motility. Int J Biochem Cell Biol 2004; 36:1890–1909.
Moissoglu K, Schwartz MA . Integrin signalling in directed cell migration. Biol Cell 2006; 98:547–555.
Suetsugu S, Takenawa T . Regulation of cortical actin networks in cell migration. Int Rev Cytol 2003; 2229:245–286.
Lauffenburger DA, Horwitz AF . Cell migration: a physically integrated molecular process. Cell 1996; 84:359–369.
Howe A, Aplin AE, Alahari SK, Juliano RL . Integrin signaling and cell growth control. Curr Opin Cell Biol 1998; 10:220–231.
Hynes RO . Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110:673–687.
Huttenlocher A, Sandborg RR, Horwitz AF . Adhesion in cell migration. Curr Opin Cell Biol 1995; 7:697–706.
Ruoslahti E . Fibronectin and its integrin receptors in cancer. Adv Cancer Res 1999; 76:1–20.
Schwartz MA, Schaller MD, Ginsberg MH . Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol 1995; 11:549–599.
Feldner JC, Brandt BH . Cancer cell motility – on the road from c-erbB-2 receptor steered signaling to actin reorganization. Exp Cell Res 2002; 272:93–108.
Zigmond SH . Beginning and ending an actin filament: control at the barbed end. Curr Top Dev Biol 2004; 63:145–188.
Chou J, Stolz DB, Burke NA, Watkins SC, Wells A . Distribution of gelsolin and phosphoinositol 4,5-bisphosphate in lamellipodia during EGF-induced motility. Int J Biochem Cell Biol 2002; 34:776–790.
Janmey PA, Stossel TP . Gelsolin-polyphosphoinositide interaction. Full expression of gelsolin-inhibiting function by polyphosphoinositides in vesicular form and inactivation by dilution, aggregation, or masking of the inositol head group. J Biol Chem 1989; 264:4825–4831.
Mere J, Chahinian A, Maciver SK, et al. Gelsolin binds to polyphosphoinositide-free lipid vesicles and simultaneously to actin microfilaments. Biochem J 2005; 386 (Part 1):47–56.
Chellaiah M, Hruska K . Osteopontin stimulates gelsolin-associated phosphoinositide levels and phosphatidylinositol triphosphate-hydroxyl kinase. Mol Biol Cell 1996; 7:743–753.
Ziehr J, Sheibani N, Sorenson CM . Alterations in cell-adhesive and migratory properties of proximal tubule and collecting duct cells from bcl-2 −/− mice. Am J Physiol Renal Physiol 2004; 287:F1154–F1163.
Sorenson CM . Interaction of bcl-2 with Paxillin through its BH4 domain is important during ureteric bud branching. J Biol Chem 2004; 279:11368–11374.
Chellaiah MA, Biswas RS, Yuen D, Alvarez UM, Hruska KA . Phosphatidylinositol 3,4,5-trisphosphate directs association of Src homology 2-containing signaling proteins with gelsolin. J Biol Chem 2001; 276:47434–47444.
Sorenson CM, Sheibani N . Focal adhesion kinase, paxillin, and bcl-2: analysis of expression, phosphorylation, and association during morphogenesis. Dev Dyn 1999; 215:371–382.
Callagy GM, Webber MJ, Pharoah PD, Caldas C . Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer 2008; 8:153.
Monni O, Joensuu H, Franssila K, et al. BCL2 overexpression associated with chromosomal amplification in diffuse large B-cell lymphoma. Blood 1997; 90:1168–1174.
Pinkas J, Martin SS, Leder P . Bcl-2-mediated cell survival promotes metastasis of EpH4 betaMEKDD mammary epithelial cells. Mol Cancer Res 2004; 2:551–556.
Ishido M, Ohtsubo R, Adachi T, Kunimoto M . Attenuation of both apoptotic and necrotic actions of cadmium by Bcl-2. Environ Health Perspect 2002; 110:37–42.
Martin SS, Leder P . Human MCF10A mammary epithelial cells undergo apoptosis following actin depolymerization that is independent of attachment and rescued by Bcl-2. Mol Cell Biol 2001; 21:6529–6536.
Lipton A, Klinger I, Paul D, Holley RW . Migration of mouse 3T3 fibroblasts in response to a serum factor. Proc Natl Acad Sci USA 1971; 68:2799–2801.
Allen PG, Janmey PA . Gelsolin displaces phalloidin from actin filaments. A new fluorescence method shows that both Ca2+ and Mg2+ affect the rate at which gelsolin severs F-actin. J Biol Chem 1994; 269:32916–32923.
Acknowledgements
We thank Dr Jau-shyong Hong (NIEHS), Dr Bob Petrovich (NIEHS), Ms Pamela D Arora (CIHR, Toronto), and Dr Yanhong Liao (Huazhong University) for materials, Dr Jeff Chou (NIEHS) for statistics, and Dr James Williams (NIEHS) for mass spectra analysis. We also thank Dr Azad Bonni (Harvard Medical School) for providing plasmid-expressing GST-bad. The Division of Intramural Research of the NIEHS, NIH supported this research through projects Z01-ES02302511 and Z01-ES2120714.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary information, Figure S1
Over expression of Bcl–2 in B6 Melanoma cells also significantly decreased cell motility. (PDF 79 kb)
Supplementary information, Figure S2
Recombinant human BCL2 protein slightly increased actin polymerization. (PDF 75 kb)
Supplementary information, Figure S3
Overexpression of BCL2 inhibited 12G10 antibody induced cell–cell adhesions in MCF–7 cells resembling the effect of Jasplakinolide. (PDF 128 kb)
Supplementary information, Data S1
Materials and Methods (PDF 118 kb)
Rights and permissions
About this article
Cite this article
Ke, H., Parron, V., Reece, J. et al. BCL2 inhibits cell adhesion, spreading, and motility by enhancing actin polymerization. Cell Res 20, 458–469 (2010). https://doi.org/10.1038/cr.2010.21
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2010.21
Keywords
This article is cited by
-
Methylation of HBP1 by PRMT1 promotes tumor progression by regulating actin cytoskeleton remodeling
Oncogenesis (2022)
-
Low cleaved caspase-7 levels indicate unfavourable outcome across all breast cancers
Journal of Molecular Medicine (2018)
-
The H1047R point mutation in p110 alpha changes the morphology of human colon HCT116 cancer cells
Cell Death Discovery (2015)
-
Bcl-2 together with PI3K p110α regulates cell morphology and cell migration
Cell Death & Disease (2015)
-
The adhesion modulation protein, AmpA localizes to an endocytic compartment andinfluences substrate adhesion, actin polymerization and endocytosis invegetative Dictyostelium cells
BMC Cell Biology (2012)