Abstract
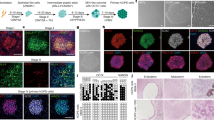
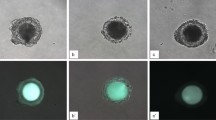
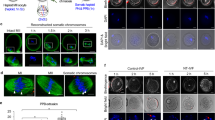
Somatic nuclei can be reprogrammed into a pluripotent state by nuclear transfer, cell fusion and expression of transcription factors. However, these reprogramming processes are very inefficient, which has greatly hindered efforts to elucidate the underlying molecular mechanisms. Here, we report a new reprogramming strategy that combines the advantages of all three reprogramming methodologies into one process. We injected nuclei from cumulus cells into intact MII oocytes. Following activation, 80% of the reconstructed embryos developed to the blastocyst stage, and tetraploid (4N) embryonic stem (ES) cell lines were generated at a rate of 30% per reconstructed oocyte. We also generated triploid (3N) ES cells after injection of somatic nuclei into activated oocytes. 4N and 3N ES cells expressed pluripotent markers and differentiated into cell types of three embryonic germ layers in vivo. Moreover, all ES cells generated histocompatible, differentiated cells after being engrafted in immunocompetent B6D2F1 mice, showing that ES cells derived from this reprogramming strategy might serve as a source of genetically tailored tissues for transplantation. Thus, we have established a simple and highly efficient reprogramming procedure that provides a system for investigating the molecular mechanisms involved in somatic reprogramming.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R . Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998; 394:369–374.
Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH . Viable offspring derived from fetal and adult mammalian cells. Nature 1997; 385:810–813.
Cowan CA, Atienza J, Melton DA, Eggan K . Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 2005; 309:1369–1373.
Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T . Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol 2001; 11:1553–1558.
Tada M, Tada T, Lefebvre L, Barton SC, Surani MA . Embryonic germ cells induce epigenetic reprogramming of somatic nucleus in hybrid cells. EMBO J 1997; 16:6510–6520.
Miller RA, Ruddle FH . Pluripotent teratocarcinoma-thymus somatic cell hybrids. Cell 1976; 9:45–55.
Do JT, Scholer HR . Nuclei of embryonic stem cells reprogram somatic cells. Stem Cells 2004; 22:941–949.
Takahashi K, Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663–676.
Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318:1917–1920.
Hochedlinger K, Plath K . Epigenetic reprogramming and induced pluripotency. Development 2009; 136:509–523.
Nagy A, Gocza E, Diaz EM, et al. Embryonic stem cells alone are able to support fetal development in the mouse. Development 1990; 110:815–821.
Ohta H, Sakaide Y, Yamagata K, Wakayama T . Increasing the cell number of host tetraploid embryos can improve the production of mice derived from embryonic stem cells. Biol Reprod 2008; 79:486–492.
Kim K, Lerou P, Yabuuchi A, et al. Histocompatible embryonic stem cells by parthenogenesis. Science 2007; 315:482–486.
Li J, Ishii T, Feinstein P, Mombaerts P . Odorant receptor gene choice is reset by nuclear transfer from mouse olfactory sensory neurons. Nature 2004; 428:393–399.
Mombaerts P . Therapeutic cloning in the mouse. Proc Natl Acad Sci USA 2003; 100 (Suppl 1):11924–11925.
Wakayama S, Suetsugu R, Thuan NV, et al. Establishment of mouse embryonic stem cell lines from somatic cell nuclei by nuclear transfer into aged, fertilization-failure mouse oocytes. Curr Biol 2007; 17:R120–121.
Gao S, McGarry M, Priddle H, et al. Effects of donor oocytes and culture conditions on development of cloned mice embryos. Mol Reprod Dev 2003; 66:126–133.
Boiani M, Eckardt S, Leu NA, Scholer HR, McLaughlin KJ . Pluripotency deficit in clones overcome by clone-clone aggregation: epigenetic complementation? EMBO J 2003; 22:5304–5312.
Wakayama T, Yanagimachi R . Effect of cytokinesis inhibitors, DMSO and the timing of oocyte activation on mouse cloning using cumulus cell nuclei. Reproduction 2001; 122:49–60.
Li J, Greco V, Guasch G, Fuchs E, Mombaerts P . Mice cloned from skin cells. Proc Natl Acad Sci USA 2007; 104:2738–2743.
Palmieri SL, Peter W, Hess H, Scholer HR . Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol 1994; 166:259–267.
Boiani M, Eckardt S, Scholer HR, McLaughlin KJ . Oct4 distribution and level in mouse clones: consequences for pluripotency. Genes Dev 2002; 16:1209–1219.
Latham KE . Early and delayed aspects of nuclear reprogramming during cloning. Biol Cell 2005; 97:119–132.
Huangfu D, Osafune K, Maehr, R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 2008; 26:1269–1275.
Kim JB, Sebastiano V, Wu G, et al. Oct4-induced pluripotency in adult neural stem cells. Cell 2009; 136:411–419.
Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 2008; 26:101–106.
Egli D, Rosains J, Birkhoff G, Eggan K . Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature 2007; 447:679–685.
Egli D, Sandler VM, Shinohara ML, Cantor H, Eggan K . Reprogramming after chromosome transfer into mouse blastomeres. Curr Biol 2009; 19:1403–1409.
McGrath J, Solter D . Inability of mouse blastomere nuclei transferred to enucleated zygotes to support development in vitro. Science 1984; 226:1317–1319.
Wakayama T, Tateno H, Mombaerts P, Yanagimachi R . Nuclear transfer into mouse zygotes. Nat Genet 2000; 24:108–109.
Matsumura H, Tada T . Cell fusion-mediated nuclear reprogramming of somatic cells. Reprod Biomed Online 2008; 16:51–56.
Hu YG, Hirasawa R, Hu JL, et al. Regulation of DNA methylation activity through Dnmt3L promoter methylation by Dnmt3 enzymes in embryonic development. Hum Mol Genet 2008; 17:2654–2664.
Acknowledgements
We thank Dr Xiang Gao of Nanjing University for providing the EGFP transgenic mice and Oct4-EGFP transgenic mice. We also thank Drs Peter Mombaerts of Max Planck Institute for Biophysics, Dangsheng Li of Shanghai Information Center for Life Sciences and Xuan Zhang of J. L. laboratory for their critical reading and suggestions made during the preparation of this paper. The study was supported by grants from the Chinese Academy of Sciences (KSCX2-YW-R-110, KSCX2-YW-R-229), the Ministry of Science and Technology (2007CB947101, 2009CB941101), the National Natural Science Foundation of China (30871430) and the Shanghai Municipal Commission for Science and Technology (07DZ22919, 08DJ1400502, 09PJ1410900).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, H., Shi, L., Zhang, S. et al. High-efficiency somatic reprogramming induced by intact MII oocytes. Cell Res 20, 1034–1042 (2010). https://doi.org/10.1038/cr.2010.97
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2010.97