Abstract
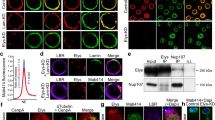
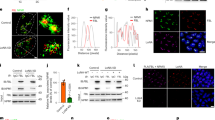
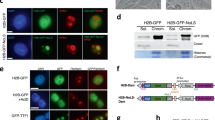
The mechanism for nuclear envelope (NE) assembly is not fully understood. Importin-β and the small GTPase Ran have been implicated in the spatial regulation of NE assembly process. Here we report that chromatin-bound NLS (nuclear localization sequence) proteins provide docking sites for the NE precursor membrane vesicles and nucleoporins via importin-α and -β during NE assembly in Xenopus egg extracts. We show that along with the fast recruitment of the abundant NLS proteins such as nucleoplasmin and histones to the demembranated sperm chromatin in the extracts, importin-α binds the chromatin NLS proteins rapidly. Meanwhile, importin-β binds cytoplasmic NE precursor membrane vesicles and nucleoporins. Through interacting with importin-α on the chromatin NLS proteins, importin-β targets the membrane vesicles and nucleoporins to the chromatin surface. Once encountering Ran-GTP on the chromatin generated by RCC1, importin-β preferentially binds Ran-GTP and releases the membrane vesicles and nucleoporins for NE assembly. NE assembly is disrupted by blocking the interaction between importin-α and NLS proteins with excess soluble NLS proteins or by depletion of importin-β from the extract. Our findings reveal a novel molecular mechanism for NE assembly in Xenopus egg extracts.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Vasu SK, Forbes DJ . Nuclear pores and nuclear assembly. Curr Opin Cell Biol 2001; 13:363–375.
Mitchell JM, Mansfeld J, Capitanio J, Kutay U, Wozniak RW . Pom121 links two essential subcomplexes of the nuclear pore complex core to the membrane. J Cell Biol 2010; 191:505–521.
Fichtman B, Ramos C, Rasala B, Harel A, Forbes DJ . Inner/Outer nuclear membrane fusion in nuclear pore assembly: biochemical demonstration and molecular analysis. Mol Biol Cell 2010; 21:4197–4211.
Hetzer MW, Wente SR . Border control at the nucleus: biogenesis and organization of the nuclear membrane and pore complexes. Dev Cell 2009; 17:606–616.
Gant TM, Wilson KL . Nuclear assembly. Annu Rev Cell Dev Biol 1997; 13:669–695.
Rasala BA, Ramos C, Harel A, Forbes DJ . Capture of AT-rich chromatin by ELYS recruits POM121 and NDC1 to initiate nuclear pore assembly. Mol Biol Cell 2008; 19:3982–3996.
Gillespie PJ, Khoudoli GA, Stewart G, Swedlow JR, Blow JJ . ELYS/MEL-28 chromatin association coordinates nuclear pore complex assembly and replication licensing. Curr Biol 2007; 17:1657–1662.
Franz C, Walczak R, Yavuz S, et al. MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep 2007; 8:165–172.
Rasala BA, Orjalo AV, Shen Z, Briggs S, Forbes DJ . ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc Natl Acad Sci USA 2006; 103:17801–17806.
Walther TC, Askjaer P, Gentzel M, et al. RanGTP mediates nuclear pore complex assembly. Nature 2003; 424:689–694.
Walther TC, Alves A, Pickersgill H, et al. The conserved Nup107-160 complex is critical for nuclear pore complex assembly. Cell 2003; 113:195–206.
Fernandez AG, Piano F . MEL-28 is downstream of the Ran cycle and is required for nuclear-envelope function and chromatin maintenance. Curr Biol 2006; 16:1757–1763.
Antonin W, Franz C, Haselmann U, Antony C, Mattaj IW . The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Mol Cell 2005; 17:83–92.
Zhang C, Goldberg MW, Moore WJ, Allen TD, Clarke PR . Concentration of Ran on chromatin induces decondensation, nuclear envelope formation and nuclear pore complex assembly. Eur J Cell Biol 2002; 81:623–633.
Hetzer M, Bilbao-Cortes D, Walther TC, Gruss OJ, Mattaj IW . GTP hydrolysis by Ran is required for nuclear envelope assembly. Mol Cell 2000; 5:1013–1024.
Ryan KJ, McCaffery JM, Wente SR . The Ran GTPase cycle is required for yeast nuclear pore complex assembly. J Cell Biol 2003; 160:1041–1053.
Zhang C, Clarke PR . Chromatin-independent nuclear envelope assembly induced by Ran GTPase in Xenopus egg extracts. Science 2000; 288:1429–1432.
Zhang C, Clarke PR . Roles of Ran-GTP and Ran-GDP in precursor vesicle recruitment and fusion during nuclear envelope assembly in a human cell-free system. Curr Biol 2001; 11:208–212.
Hutchins JR, Moore WJ, Clarke PR . Dynamic localisation of Ran GTPase during the cell cycle. BMC Cell Biol 2009; 10:66.
Liu Q, Yu J, Zhuo X, Jiang Q, Zhang C . Pericentrin contains five NESs and an NLS essential for its nucleocytoplasmic trafficking during the cell cycle. Cell Res 2010; 20:948–962.
Jiang Q, Lu Z, Zhang C . Role of Ran GTPase in cell cycle regulation. Chinese Sci Bull 2004; 49:535–541.
Zhang C, Hutchins JR, Muhlhausser P, Kutay U, Clarke PR . Role of importin-beta in the control of nuclear envelope assembly by Ran. Curr Biol 2002; 12:498–502.
Clarke PR, Zhang C . Spatial and temporal coordination of mitosis by Ran GTPase. Nat Rev Mol Cell Biol 2008; 9:464–477.
Harel A, Chan RC, Lachish-Zalait A, Zimmerman E, Elbaum M, Forbes DJ . Importin beta negatively regulates nuclear membrane fusion and nuclear pore complex assembly. Mol Biol Cell 2003; 14:4387–4396.
Hachet V, Kocher T, Wilm M, Mattaj IW . Importin alpha associates with membranes and participates in nuclear envelope assembly in vitro. EMBO J 2004; 23:1526–1535.
Gorlich D, Vogel F, Mills AD, Hartmann E, Laskey RA . Distinct functions for the two importin subunits in nuclear protein import. Nature 1995; 377:246–248.
Clarke PR, Zhang C . Spatial and temporal control of nuclear envelope assembly by Ran GTPase. Symp Soc Exp Biol 2004; 56:193–204.
Ma Y, Cai S, Lv Q, et al. Lamin B receptor plays a role in stimulating nuclear envelope production and targeting membrane vesicles to chromatin during nuclear envelope assembly through direct interaction with importin beta. J Cell Sci 2007; 120:520–530.
Lu X, Shi Y, Lu Q, et al. Requirement for lamin B receptor and its regulation by importin {beta} and phosphorylation in nuclear envelope assembly during mitotic exit. J Biol Chem 2010; 285:33281–33293.
Laskey RA, Mills AD, Philpott A, Leno GH, Dilworth SM, Dingwall C . The role of nucleoplasmin in chromatin assembly and disassembly. Philos Trans R Soc Lond B Biol Sci 1993; 339:263–269; discussion 268–269.
Philpott A, Leno GH . Nucleoplasmin remodels sperm chromatin in Xenopus egg extracts. Cell 1992; 69:759–767.
Lu Z, Zhang C, Zhai Z . Nucleoplasmin regulates chromatin condensation during apoptosis. Proc Natl Acad Sci USA 2005; 102:2778–2783.
Rice P, Garduno R, Itoh T, Katagiri C, Ausio J . Nucleoplasmin-mediated decondensation of Mytilus sperm chromatin. Identification and partial characterization of a nucleoplasmin-like protein with sperm-nuclei decondensing activity in Mytilus californianus. Biochemistry 1995; 34:7563–7568.
Yang CH, Lambie EJ, Snyder M . NuMA: an unusually long coiled-coil related protein in the mammalian nucleus. J Cell Biol 1992; 116:1303–1317.
Merdes A, Ramyar K, Vechio JD, Cleveland DW . A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 1996; 87:447–458.
Philpott A, Leno GH, Laskey RA . Sperm decondensation in Xenopus egg cytoplasm is mediated by nucleoplasmin. Cell 1991; 65:589–578.
Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA . Importin a: a multipurpose nuclear-transport receptor. Trends Cell Biol 2004; 14:505–514.
Banuelos S, Hierro A, Arizmendi JM, Montoya G, Prado A, Muga A . Activation mechanism of the nuclear chaperone nucleoplasmin: role of the core domain. J Mol Biol 2003; 334:585–593.
Hierro A, Arizmendi JM, Banuelos S, Prado A, Muga A . Electrostatic interactions at the C-terminal domain of nucleoplasmin modulate its chromatin decondensation activity. Biochemistry 2002; 41:6408–6413.
Dingwall C, Dilworth SM, Black SJ, Kearsey SE, Cox LS, Laskey RA . Nucleoplasmin cDNA sequence reveals polyglutamic acid tracts and a cluster of sequences homologous to putative nuclear localization signals. EMBO J 1987; 6:69–74.
Dingwall C, Sharnick SV, Laskey RA . A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell 1982; 30:449–458.
Wiese C, Wilde A, Moore MS, Adam SA, Merdes A, Zheng Y . Role of importin-β in coupling Ran to downstream targets in microtubule assembly. Science 2001; 291:653–656.
Gruss OJ, Carazo-Salas RE, Schatz CA, et al. Ran induces spindle assembly by reversing the inhibitory effect of importin α on TPX2 activity. Cell 2001; 102:83–93.
Bilbao-Cortés D, Hetzer M, Längst G, Becker PB, Mattaj IW . Ran binds to chromatin by two distinct mechanisms. Curr Biol 2002; 12:1151–1156.
Hughes M, Zhang C, Avis JM, Hutchison CJ, Clarke PR . The role of the ran GTPase in nuclear assembly and DNA replication: characterisation of the effects of Ran mutants. J Cell Sci 1998; 111:3017–3026.
Hartl P, Olson E, Dang T, Forbes DJ . Nuclear assembly with lambda DNA in fractionated Xenopus egg extract: an unexpected role for glycogen in formation of a higher order chromatin intermediate. J Cell Biol 1994; 124:235–248.
Acknowledgements
We gratefully thank all the other members in our laboratories for helpful comments. We thank Dr TK Tang for providing the plasmid containing full-length NuMA cDNA. This work was supported by grants from the State Key Basic Research and Development Plan (2010CB833705) and the National Natural Science Foundation of China (30900726, 31071188, 31030044 and 90913021) to CZ, and by a Royal Society-Wolfson Research Merit Award to PRC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Q., Lu, Z., Liu, Q. et al. Chromatin-bound NLS proteins recruit membrane vesicles and nucleoporins for nuclear envelope assembly via importin-α/β. Cell Res 22, 1562–1575 (2012). https://doi.org/10.1038/cr.2012.113
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2012.113