Abstract
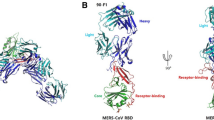
The newly-emerging Middle East respiratory syndrome coronavirus (MERS-CoV) can cause severe and fatal acute respiratory disease in humans. Despite global efforts, the potential for an associated pandemic in the future cannot be excluded. The development of effective counter-measures is urgent. MERS-CoV-specific anti-viral drugs or vaccines are not yet available. Using the spike receptor-binding domain of MERS-CoV (MERS-RBD) to immunize mice, we identified two neutralizing monoclonal antibodies (mAbs) 4C2 and 2E6. Both mAbs potently bind to MERS-RBD and block virus entry in vitro with high efficacy. We further investigated their mechanisms of neutralization by crystallizing the complex between the Fab fragments and the RBD, and solved the structure of the 4C2 Fab/MERS-RBD complex. The structure showed that 4C2 recognizes an epitope that partially overlaps the receptor-binding footprint in MERS-RBD, thereby interfering with the virus/receptor interactions by both steric hindrance and interface-residue competition. 2E6 also blocks receptor binding, and competes with 4C2 for binding to MERS-RBD. Based on the structure, we further humanized 4C2 by preserving only the paratope residues and substituting the remaining amino acids with the counterparts from human immunoglobulins. The humanized 4C2 (4C2h) antibody sustained similar neutralizing activity and biochemical characteristics to the parental mouse antibody. Finally, we showed that 4C2h can significantly abate the virus titers in lungs of Ad5-hCD26-transduced mice infected with MERS-CoV, therefore representing a promising agent for prophylaxis and therapy in clinical settings.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
Accessions
GenBank/EMBL/DDBJ
Protein Data Bank
References
Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA . Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367:1814–1820.
Chan JF, Li KS, To KK, Cheng VC, Chen H, Yuen KY . Is the discovery of the novel human betacoronavirus 2c EMC/2012 (HCoV-EMC) the beginning of another SARS-like pandemic? J Infect 2012; 65:477–489.
Zhong NS, Zheng BJ, Li YM, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet 2003; 362:1353–1358.
Health Protection Agency UKNCIt. Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill 2013; 18:20427.
Breban R, Riou J, Fontanet A . Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet 2013; 382:694–699.
Su S, Wong G, Liu Y, Gao GF, Li S, Bi Y . MERS in South Korea and China: a potential outbreak threat? Lancet 2015; 385:2349–2350.
Lu G, Hu Y, Wang Q, et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature 2013; 500:227–231.
Weiss SR, Navas-Martin S . Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev 2005; 69:635–664.
Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S . The spike protein of SARS-CoV-a target for vaccine and therapeutic development. Nat Rev Microbiol 2009; 7:226–236.
Masters PS . The molecular biology of coronaviruses. Adv Virus Res 2006; 66:193–292.
Raj VS, Mou HH, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013; 495:251–254.
Graham RL, Donaldson EF, Baric RS . A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol 2013; 11:836–848.
Lu G, Wang Q, Gao GF . Bat-to-human: spike features determining 'host jump' of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol 2015; 23:468–478.
Wang N, Shi X, Jiang L, et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res 2013; 23:986–993.
Gao J, Lu G, Qi J, et al. Structure of the fusion core and inhibition of fusion by a heptad repeat peptide derived from the S protein of Middle East respiratory syndrome coronavirus. J Virol 2013; 87:13134–13140.
Lu L, Liu Q, Zhu Y, et al. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat Commun 2014; 5:3067.
Du L, Zhao G, Kou Z, et al. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J Virol 2013; 87:9939–9942.
Du L, Kou Z, Ma C, et al. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: Implication for developing therapeutics and vaccines. PLoS One 2013; 8:e81587.
Song F, Fux R, Provacia LB, et al. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J Virol 2013; 87:11950–11954.
Tang XC, Agnihothram SS, Jiao Y, et al. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc Natl Acad Sci USA 2014; 111:E2018–E2026.
Jiang L, Wang N, Zuo T, et al. Potent neutralization of MERS-CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein. Sci Transl Med 2014; 6:234ra259.
Pascal KE, Coleman CM, Mujica AO, et al. Pre- and postexposure efficacy of fully human antibodies against Spike protein in a novel humanized mouse model of MERS-CoV infection. Proc Natl Acad Sci USA 2015; 112:8738–8743.
Zhao J, Li K, Wohlford-Lenane C, et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci USA 2014; 111:4970–4975.
Falzarano D, de Wit E, Rasmussen AL, et al. Treatment with interferon-alpha2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med 2013; 19:1313–1317.
Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis 2014; 14:1090–1095.
D'Souza P, Walker G . Spotlight on chronic lymphocytic leukemia: A Pharma Matters report. Drugs Today (Barc) 2014; 50:485–501.
Murray J, Saxena S, Sharland M . Preventing severe respiratory syncytial virus disease: passive, active immunisation and new antivirals. Arch Dis Child 2014; 99:469–473.
Jacob J, Kirova YM . Locoregional breast radiotherapy and concurrent treatment with trastuzumab. Bull Cancer 2014; 101:40–51.
Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47–53.
Bermingham A, Chand MA, Brown CS, et al. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill 2012; 17:20290.
Liu J, Lester P, Builder S, Shire SJ . Characterization of complex formation by humanized anti-IgE monoclonal antibody and monoclonal human IgE. Biochemistry 1995; 34:10474–10482.
Muller YA, Chen Y, Christinger HW, et al. VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 angstrom resolution and mutational analysis of the interface. Structure 1998; 6:1153–1167.
Faelber K, Kirchhofer D, Presta L, Kelley RF, Muller YA . The 1.85 Å resolution crystal structures of tissue factor in complex with humanized Fab D3h44 and of free humanized Fab D3h44: revisiting the solvation of antigen combining sites. J Mol Biol 2001; 313:83–97.
Karpusas M, Lucci J, Ferrant J, et al. Structure of CD40 ligand in complex with the Fab fragment of a neutralizing humanized antibody. Structure 2001; 9:321–329.
Ying T, Du L, Ju TW, et al. Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies. J Virol 2014; 88:7796–7805.
Huang W, Samanta M, Crawford SE, et al. Identification of human single-chain antibodies with broad reactivity for noroviruses. Protein Eng Des Sel 2014; 27:339–349.
Houimel M . The analysis of VH and VL genes repertoires of Fab library built from peripheral B cells of human rabies virus vaccinated donors. Hum Immunol 2014; 75:745–755.
Avnir Y, Tallarico AS, Zhu Q, et al. Molecular signatures of hemagglutinin stem-directed heterosubtypic human neutralizing antibodies against influenza A viruses. PLoS Pathog 2014; 10:e1004103.
Li M, Gao F, Mascola JR, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 2005; 79:10108–10125.
Chan KH, Chan JF, Tse H, et al. Cross-reactive antibodies in convalescent SARS patients' sera against the emerging novel human coronavirus EMC (2012) by both immunofluorescent and neutralizing antibody tests. J Infect 2013; 67:130–140.
Zheng BJ, Chan KW, Lin YP, et al. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl Acad Sci USA 2008; 105:8091–8096.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program; 2015CB910503), the National Science and Technology Major Project of China (2013ZX10004608-002 and 2014ZX10004001-006), the National Natural Science Foundation of China (NSFC; 31400154 and 81461168030), the National Institutes of Health, USA (AI60699) and Hong Kong Research Grants Council (NHKU728/14). We acknowledge Dr Kwok-Hung Chan and Dr Jasper FW Chan for their technical support and the staff at the Shanghai Synchrotron Radiation Facility (SSRF-beam line 17U). We would be grateful to Yuanyuan Chen (Institute of Biophysics, CAS) for technical help with Biacore experiments. GFG is a leading principal investigator of the NSFC Innovative Research Group (8132106).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Y., Wan, Y., Liu, P. et al. A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein. Cell Res 25, 1237–1249 (2015). https://doi.org/10.1038/cr.2015.113
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2015.113
Keywords
This article is cited by
-
The structure of a novel antibody against the spike protein inhibits Middle East respiratory syndrome coronavirus infections
Scientific Reports (2022)
-
Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases
Nature Medicine (2021)
-
Smarter cures to combat COVID-19 and future pathogens: a review
Environmental Chemistry Letters (2021)
-
Analysis of the SARS-CoV-2 spike protein glycan shield reveals implications for immune recognition
Scientific Reports (2020)
-
Development of an antibody-dependent cellular cytotoxicity reporter assay for measuring anti-Middle East Respiratory Syndrome antibody bioactivity
Scientific Reports (2020)