Precision BioSciences is a clinical-stage gene-editing company focused on the development of cell and gene therapies to treat cancer and genetic causes of disease. The company’s pipeline of products based on allogeneic chimeric antigen receptor (CAR)-T cells are engineered ex vivo using the ARCUS platform to optimize donor cells and thereby improve clinical performance. The lead candidate azer-cel (PBCAR0191) is in a phase 1/2a clinical trial as a potential first-in-class cell therapy for patients with CD19+ B cell acute lymphoblastic leukemia who have previously received a CAR-T treatment and relapsed. Two more candidates, PBCAR19B and PBCAR269A, are also in the clinic as potential best-in-class CAR-T therapies for relapsed or refractory patients with non-Hodgkin lymphoma and multiple myeloma, respectively.
For its wholly owned in vivo programs, Precision is developing an ARCUS nuclease that targets chronic hepatitis B covalently closed circular DNA. For its primary hyperoxaluria type 1 program, Precision is developing an ARCUS nuclease to knock out the hydroxyacid oxidase 1 gene, HAO1, to treat the severe and potentially fatal accumulation of kidney and urinary-tract stones. Precision is also using its ARCUS platform to develop a treatment to edit PCSK9, a gene that regulates the levels of low-density lipoprotein (LDL) cholesterol. In preclinical studies, and three years after the initial treatment, which triggered a reduction of up to 60% in serum LDL, the animals continued to exhibit low LDL levels, suggesting a long-term effect from the one-time treatment. The company also has multiple development programs underway with pharma partners, including programs for Duchenne muscular dystrophy and sickle-cell disease.
The ARCUS advantage
“Over the past two decades, the interest and investment in new therapeutic modalities such as gene editing to create medicines with transformational treatment options has opened new possibilities to tackle targets and indications that have long been considered incurable,” said Derek Jantz, co-founder and CSO and strategy head of Precision. “Precision’s ARCUS gene-editing platform is derived from a naturally occurring gene-editing enzyme with unique specificity, precision, and delivery characteristics optimal for clinical application.”
Genome-editing technologies enable precise editing of the DNA of a living organism, opening up the possibility of correcting genetic problems at their source. The ARCUS gene-editing platform is derived from the nuclease I-CreI of the green alga Chlamydomonas reinhardtii. This nuclease evolved naturally to perform a single, highly specific DNA edit. The high specificity exhibited by I-CreI derives from its recognition of a long, 22-base-pair DNA target sequence and the integrated structure of its DNA-binding and DNA-cutting domains. The long recognition sequence enhances the ability to identify a unique target within genes of interest, and the integration of the DNA-binding and DNA-cutting domains offers an additional safety check, because conformational activation of the nuclease requires binding to a 22-base-pair sequence (Fig. 1).
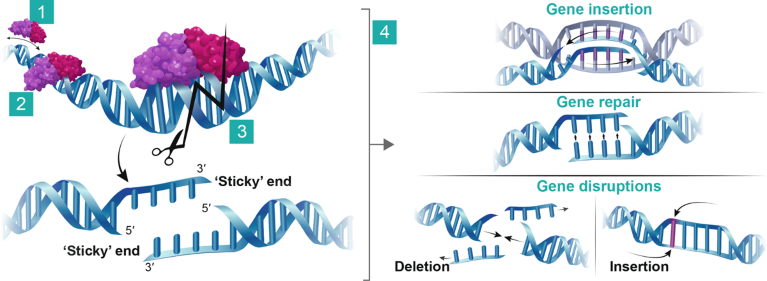
Fig. 1 | The ARCUS gene-editing platform. (1) The ARCUS nuclease scans DNA for the target site. (2) The ARCUS nuclease binds to the target site. (3) The target DNA sequence is cut, creating a ‘sticky’ 4-base 3’ overhang. (4) The cut target site is repaired via homology-directed repair (HDR) or non-homologous end joining (NHEJ). If a template DNA sequence is supplied, the DNA can be precisely repaired or altered (including gene insertion) via HDR. Without a template DNA sequence, the ‘sticky’ ends are repaired via NHEJ; however, this process can lead to insertions and/or deletions, both of which can lead to gene disruption.
Each ARCUS nuclease is generated through an iterative protein-engineering process to optimize on-target binding and cutting activity while minimizing off-target editing. This process yields highly specific nucleases, including those capable of differentiating between target sequences that differ by just a single base pair. Additionally, the cutting activity can be customized to match the type of delivery, the concentration, and the desired activity window in the target cells. And finally, the small size of the ARCUS nuclease—only 364 amino acids—enables delivery to tissues and cells using a variety of viral and non-viral delivery technologies, including those using adeno-associated virus (AAV) and lipid nanoparticles (LNP).
Strategic partnering for cell and gene therapies
The rapidly growing fields of cell and gene therapy are transforming the standard of care in cancer and genetic disease. However, they bring significant challenges, such as delivery, clinical safety, manufacturing and commercial considerations. Overcoming these challenges requires strategic alliances between players in the industry.
Precision is collaborating with multiple global healthcare leaders to bring its deep gene-editing knowledge together with premier drug discovery, development and commercial experience to accelerate work aimed at solving genetic diseases with unique editing challenges. In collaboration with Lilly, for example, Precision is using a pair of ARCUS nucleases to simultaneously cut two target sites in the Duchenne muscular dystrophy-associated dystrophin gene, with the goal of deleting 500-kilobase pairs that include disease-associated exons 45 to 55. For its most recently signed research collaboration, with Novartis, Precision is leveraging ARCUS’ uniquely evolved capacity for gene insertion to develop a potential one-time transformative treatment option for diseases including certain hemoglobinopathies, such as sickle-cell disease and beta thalassemia.
“Partnering is a cornerstone of our company’s growth and success,” said Cindy Atwell, CBO of Precision. “We continue to look for strategic research and licensing opportunities where we can bring together the power of the ARCUS genome-editing platform with targets of interest and drug development expertise for cell and gene therapies.”


