Food allergies affect around 220 million people worldwide, and their incidence is on the rise. The impact can range from simply unpleasant effects to life-threatening anaphylaxis. Patients also appear to be at greater risk of inflammatory bowel disease (IBD). Adversely impacting quality of life, IBD affects up to 10 million worldwide. The only potentially curative treatment, allergen immunotherapy (AIT), often fails to bring convincing real-life outcomes.
Enterome’s Mimicry approach harnesses the gut’s natural tolerance pathway to halt harmful inflammatory responses in antigen-mediated allergic and autoimmune diseases.
Mimics-based food-allergy treatments
Co-founded in 2012 in Paris, France, by CEO Pierre Belichard, Enterome has a unique discovery platform and in-house-developed database with potential through several pharma applications. While the company’s most advanced programs are running in oncology, Enterome has partnered programs in allergies and autoimmune diseases, in which T cells mistakenly react against specific food or self-antigens.
Around 70% of immune cells that normally act as sentinels against pathogens, including memory T cells and regulatory T cells (Treg cells), reside in the lining of the gut. This proximity means that the microbiota—the community of microorganisms that live in symbiosis with the human body—has a significant impact on the activity of the immune system.
“The human gut microbiome is a reservoir of millions of bacterial proteins and peptides. Many of these bear striking similarity to food allergens. Our microbiome and the associated peptides have co-evolved with us. In our early years they act to train our immune system and tolerize us against proteins found in food. People with allergies are missing these ‘training’ peptides,” explained Christophe Bonny, CSO of Enterome. “Our approach to food allergies, known as AllerMimics, utilizes these naturally occurring allergen mimics to achieve tolerance.”
Enterome’s bacteria-derived peptides teach the immune system to tolerate the selected antigen thereby suppressing the pathology. Upon subsequent exposure, the body no longer mounts an immune response toward the tissue expressing this antigen.
Administration of AllerMimics is conceptually similar to vaccination: a vaccine helps the immune system to recognize and target a pathogen, whereas this peptide-based therapy trains the immune system to recognize and tolerate harmless antigens—without affecting its function in any other way. As these are synthetically produced proteins and peptides, they are relatively cheap to manufacture.
Building technology from commensal bacteria
The Enterome database comprises more than 20 million full-length gut-microbiome peptides and proteins. “The vast majority of these are rare, with hundreds of thousands being small and constrained proteins and peptides displaying hormone-like features, and only a limited number of microbiome genomes are shared between people,” explained Bonny.
The process of drug discovery at Enterome begins with the gut and these immune cells (Fig. 1). The company chooses targets that interact with gut-wall cells and that play a significant role in disease through the modulation of T cells (oncology, food allergy and autoimmunity), or the production of cytokines and hormones (gastrointestinal inflammatory and immune diseases). The subset libraries are screened using peripheral blood mononuclear cells (PBMCs) to identify potential lead drugs against the targets.
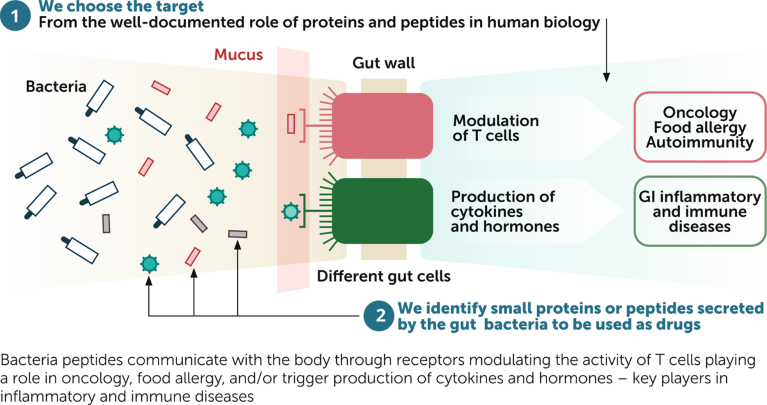
Fig. 1 | Enterome’s approach to drug discovery. Decoding the interactions between gut microbiome proteins and the immune system. GI, gastrointestinal.
Addressing unmet needs in food allergies
In July 2022, Enterome signed an exclusive agreement with Nestlé Health Science to co-develop its lead EndoMimics clinical candidate. A local inducer of interleukin-10 (IL-10), EB1010 is conceived as an oral gastro-resistant treatment for food allergies and IBD. EB1010 is due to enter an ulcerative colitis clinical trial in 2023.
The two partners will also use the Mimicry platform to create a pipeline of AllerMimics that mimic food allergens to build immune tolerance, and EndoMimics that have the potential to reduce inflammation caused by food allergies.
“We had existing links with Nestlé Health Science. Seeing the positive clinical data we generated in oncology, they approached us suggesting a collaboration in food allergies. As well as providing us with funding and support to develop our pipeline, this partnership exemplifies the potential of our highly productive Mimicry platform that has already demonstrated clinical efficacy with our OncoMimics immunotherapies for cancer,” said Bonny.
Tumor-antigen mimics as cancer treatments
Enterome’s lead OncoMimics candidate, EO2401, mimics three key tumor antigens that are highly expressed by brain tumors and shows promising efficacy in phase 1/2 clinical trials in patients with glioblastoma and adrenal tumors, in combination with checkpoint inhibitors. EO2463 is also in phase 1/2 for non-Hodgkin lymphomas. And the EO2040 candidate is due to start phase 2 by the end of 2022 for colorectal cancer with circulating tumor DNA (ctDNA)-defined minimal residual disease. “We plan to develop agents in our OncoMimics pipeline to phase 2 proof-of-concept, and then work with a partner for further development and commercialization,” explained Bonny.


