The probability of technical and regulatory success (PTRS) in drug development is around 8%1. The highest impact is the 36% probability of success in phase 2, impacted by limited efficacy. To increase efficacy success and thereby patient health and commercial outcomes, pharma must improve the accuracy and speed of decisions by enabling evidence generation on disease and treatment at speed and at scale.
CytoReason is a technology company focused on exactly that. Its mission is to transform medicine from trial and error to predictable practice by developing the most accurate and useful computational disease models to improve the probability of efficacy success. Its computational disease models and data analytics platform products are trusted, and the predicted results are validated, by top pharma.
Increase understanding of disease biology
Pharma companies must generate a mechanistic understanding of disease and target biology to mitigate the risk of drug discovery failures. Therapeutic leaders are accountable for making many ‘right’ portfolio decisions at speed, but significant complexities result in decisions that negatively impact the probability of efficacy success and result in clinical failures and impaired outcomes.
“In addition, most drugs for chronic diseases are efficacious in only 30–40% of the patient population,” said Yehuda Chowers, CytoReason’s chief medical officer. “A limited understanding of the disease course complicates the ability to predict what’s happening to what patient and what treatment is expected to help. Much of this is due to people being very hetero-geneous because of different genetic backgrounds and exposure to environmental factors.”
The practice of medicine is still mostly trial and error where a doctor selects the ‘right’ treatment for a patient, mostly informed by clinical features without considering the mechanistic biological features and context. Accumulating evidence suggests ineffective drugs can diminish responses to later lines of therapy.
Enable differentiated evidence generation
To stay ahead of the competition, pharma companies must generate evidence related to disease and target biology at high quality and at pace. Rapidly maturing computational capabilities are required to enable a company’s scientists to benefit from ever-increasing volumes of data types and sources, and generate advanced disease, treatment, and patient population insights at scale. However, pharma’s tendency to build in-house or to experiment in silos may reduce the scalability of evidence generation, and its ability to keep up with novel and innovative computational approaches at speed while supporting drug development, thereby increasing portfolio and commercial risks. Companies that leverage an analytical platform built on advanced computational technologies and empower their talent with differentiated disease and target biology insights at speed and at scale will outperform the competition.
Impact potential
The key question for pharma is “How might we improve the accuracy and speed of our therapeutic decision-making using disease, treatment, and patient population evidence that maximizes clinical and commercial impact while minimizing risk of failures?”. As data accumulates, the way to tackle this question asks for a platform approach. When speed and accuracy are improved, the clinical and commercial impact are expected to be significant because of improved strategic decision quality and R&D acceleration, asset and portfolio return on investment (ROI), and commercial performance.
First, strategic decision quality and R&D acceleration are increased, for example, by focusing on prioritized indications and subgroups with a stronger biological rationale, or by faster go/no-go asset development decision making due to mechanism and disease fit validation.
Second, asset and portfolio ROI are strengthened by shortened time to market and a longer period of market exclusivity resulting from patent rights, by avoiding late-stage asset failures, by steering investments earlier towards better biological fits, or by unlocking higher peak sales potential from expanded subpopulations/indication sequencing.
Third, optimal commercial performance can be achieved, for example, by launching into high responder patient segments identified by subpopulation-specific biomarkers, or by stronger competitive differentiation based on positioning versus standard of care (SOC) based on mechanism insights.
“The yearly impact potential across these categories is expected to be significant when realized for one asset, let alone when applied to the portfolios of one or more therapeutic areas at a top pharma, which is estimated to be in the billions of dollars,” said Nicole van Poppel, CytoReason’s chief business officer.
CytoReason’s differentiated approach
To increase disease biology understanding and enable pharma talent with evidence generation at speed and scale, CytoReason brings a unique and proven approach to the market that has been recognized by tech and science leaders for its differentiation.
Founded in 2016, CytoReason committed from day one to disrupt the industry through a state-of-the-art deep-science technological platform that has the ability to scale and inform with the best data-driven evidence for every drug (candidate). CytoReason has created standardized computational models that simulate the disease molecular environment by integrating a range of complex biological multi-modal data, including RNA, single cell and genetic, and clinical data, with the ability to enrich with other data types such as proteomics, spatial, and epigenetics. The company has comprehensively modeled cells, cell states, and cell interaction networks, and how they change in response to drugs and over time, to provide a standardized framework for different diseases and subpopulations, across therapeutic areas. Its data analytics platform provides tools for the user to design a biologically accurate asset signature, introduce it into the simulated environment, and test for efficacy and how these would be reflected in the clinical features of the simulated population. CytoReason’s growing portfolio of tissue-based and cell-centered disease models covers many immune mediated diseases and cancers, such as ulcerative colitis and non-small cell lung carcinoma, with disease models being added and matured over time.
Customers can fine tune CytoReason’s models to create customer specific models by ingesting proprietary data for enriched insights to impact competitive differentiation. Moreover, CytoReason’s data partnerships allow customers to benefit from proprietary and high-quality sources of “deep data” to further enrich the insights.
Whereas other pharma AI companies have pursued a techbio angle, CytoReason is a technology company that doesn’t develop its own drugs. This allows CytoReason to focus all of its investments and expertise on continuously redefining the boundaries of immune-related systems biology and computational approaches through innovation, which regularly gets published in top tier journals such as Cell and Nature. These innovations are turned into next-generation capabilities, continually improving its industry-leading disease modeling capabilities, and maximizing the ability for its customers to answer questions that shape clinical and commercial success. Its differentiation is recognized by technology and scientific market leaders such as NVIDIA, Thermo Fisher Scientific and Pfizer, which, in 2024, collectively committed to investing $80m into CytoReason as part of series B funding2.
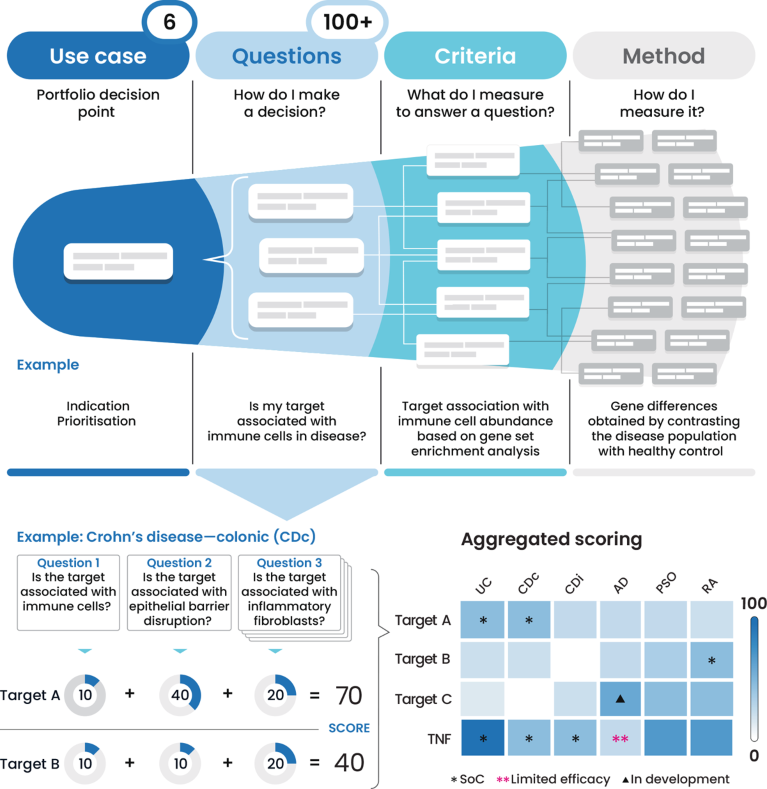
Fig. 1 | CytoReason’s criteria framework and approach to insights generation. AD, atopic dermatitis; CDc, Crohn’s disease—colonic; CDi, Crohn’s disease—ileum; PSO, psoriasis; RA, rheumatoid arthritis; SoC, standard of care; TNF, tumour necrosis factor; UC, ulcerative colitis.
CytoReason-enabled and proven insights
Insights enabled by CytoReason’s computational disease models and data analytics platform inform the following set of portfolio decisions (use cases), each of which are critical to the probability of efficacy success:
• Disease mechanisms: Find molecular features associated with disease trajectory.
• Therapeutics mechanism: Define links between the drug effect on the target and the disease direction.
• Target prioritization: Define the space within the disease where a customer’s asset is expected to have the most effect.
• Indication prioritization/expansion: Identify what (additional) indication mechanism of action (MoA) is most likely to be affected by an asset.
• Subpopulation prioritization: Characterize meaningful patient groups to inform decision making, such as definition of inclusion criteria and identification of biomarkers.
• Mono/combo therapy positioning: Differentiate the MoA and molecular score of an asset to SOC, and/or identify complementary mechanisms that may break the efficacy ceiling.
For each use case, CytoReason’s data analytics platform and its criteria framework break down questions that impact research and development (R&D) inflection points, such as target or indication prioritization (Fig. 1). Evidence is available to address hundreds of biological questions that can be selected based on the relevant clinical and biological context. Each question can be answered using multimodal data layers that get aggregated into a single metric, which is then weighted to create a single explainable score value per target. By comparing scores across indications and targets (see anonymized but real targets and TNF in heatmap in Fig. 1), users identify new opportunities for indication expansion and inform critical portfolio decisions.
CytoReason’s platform enables users to evaluate how different drug mechanisms complement each other. Changes in target effect over time in treated patients highlight which target mechanisms show similar dynamics to the SOC (Fig. 2, target B), suggesting an amplifying effect, and which targets display different dynamics (Fig. 2, target A), suggesting a complementary effect.
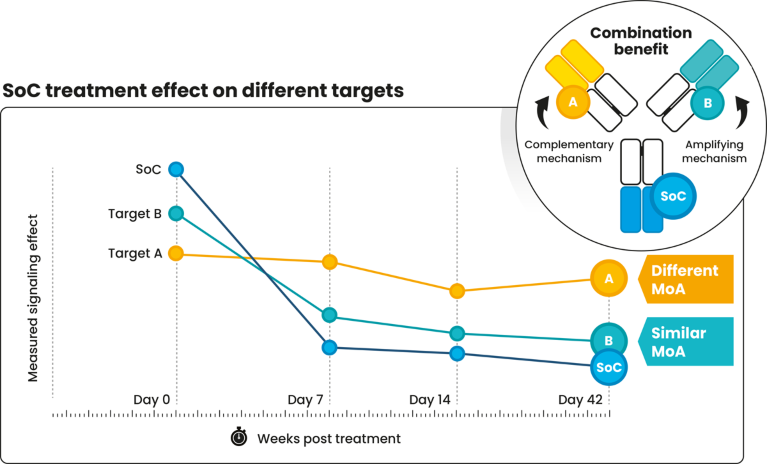
Fig. 2 | A combination therapy positioning example. MoA, mechanism of action; SoC, standard of care.
“CytoReason’s disease models give companies a ‘molecular yardstick’ to enable quantitative comparisons across drugs, indications, and subpopulations, allowing the user to weigh molecular and clinical criteria using explainable AI that makes it explainable,” said Shai Shen-Orr, CytoReason’s co-founder and chief scientist. “Biological, therapeutic, commercial, computational, and business development leaders all look at the same molecular and biological data in the platform, bridging molecular insights for scientists and actionable clinical characteristics for trial designers.”
Trusted and proven: scaling the impact enabled by CytoReason’s products
A rapidly increasing number of top 15 pharma is leveraging and scaling CytoReason’s disease modeling capabilities3,4, a testament to the differentiated products and value proposition. Multiple customers have validated CytoReason-enabled insights using complementary approaches (for example, clinical data), strengthening confidence in the products.
CytoReason builds customer trust in its products using a phased approach. Initially, the customer works side by side with CytoReason to access the platform and generate insights at quality and speed, and over time, scale use across therapeutic areas, diseases, portfolio decisions, and functions, and expand the data to be ingested and integrated.
CytoReason is continuing to improve and expand its products to stay ahead of the never-ending proliferation of data sources, data types and data science approaches to equip its customers with evidence to impact patient health and commercial outcomes.
Leaders tasked with informing or making difficult portfolio decisions benefit from insights enabled by CytoReason’s products to shift the dynamic from trial and error to predictable. Now is the moment to explore how your organization can benefit.


