The Korea Drug Development Fund (KDDF), the largest non-dilutive funding organization supporting Korea’s pharmaceutical industry, is intensifying its focus on novel treatments for neurodegenerative disorders. KDDF provides >$150 million annually to support innovative drug development pipelines across the research and development (R&D) continuum—from discovery through to clinical trials.
Of the 496 pipelines funded since 2021, 42 focus on neuroscience modalities including small molecules (48% of programs), gene-based therapies (29%), and emerging approaches such as recombinant proteins, targeted protein degraders (TPDs), antibodies, and nanoparticles (Fig. 1). These choices reflect both global development trends and the complexity of treating neurodegenerative diseases.
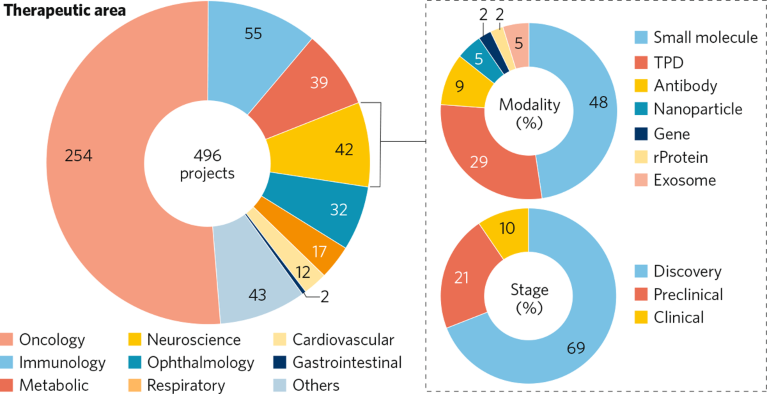
Fig. 1 | A spotlight on KDDF’s neuroscience development pipeline as of June 2025. To find out more about any of these projects please contact KDDF. rProtein, recombinant protein; TPD, targeted protein degraders.
Upstream investment and early-stage innovation are central to KDDF’s strategy for translating Korean science into first-in-class therapies, with 69% of programs in discovery and 21% in preclinical development. To amplify global impact, KDDF fosters international collaboration by supporting co-development opportunities and early-stage licensing discussions positioning Korean biotech as a key player in the neurotherapeutics landscape. Three examples are discussed here.
Zincure: treating neurodegenerative disease
Losses of degradative function in lysosomes and autophagy flux are frequently seen in aging and neurodegenerative diseases, especially amyotrophic lateral sclerosis (ALS). This dysfunction can lead to the accumulation and aggregation of toxic proteins. Zincure is developing ZCS1, a peptide oligomer engineered for endocytic uptake and restoration of lysosomal function, as a novel disease-modifying approach for neurodegenerative diseases.
ZCS1 promotes v-ATPase assembly to acidify lysosomes, thereby reactivating enzymatic function, and activates TFEB, a master regulator of lysosomal biogenesis, enhancing nuclear translocation and increasing lysosome production. These actions restore autophagic flux and facilitate the breakdown of disease-linked protein aggregates, notably TAR DNA-binding protein 43 (TDP-43), which accumulates in >95% of ALS patients regardless of genetic background.
Preclinical data from the superoxide dismutase (SOD1)-G93A mouse model suggest that subcutaneous ZCS1 administration extends survival by >3 weeks and reduces blood levels of neurofilament light chain (NfL), a sensitive biomarker for neurodegeneration, by ~50%. ZCS1 also significantly decreases TDP-43 aggregates, as confirmed by Western blot and immunohistochemistry. With desirable pharmacodynamic properties and high subcutaneous bioavailability, ZCS1 shows promise as a disease-modifying therapy. Zincure is progressing towards good laboratory practice (GLP) toxicology studies in 2026, with a first-in-human phase 1 clinical trial expected in late 2027.
PRG S&Tech Inc.: Amisodin to treat ALS
PRG S&Tech Inc., a biopharma company with a focus on rare genetic disorders, is developing Amisodin, a first-in-class, small-molecule oral drug for ALS. Amisodin has been engineered to selectively bind the trimeric form of mutant, cytotoxic SOD1 and halt downstream protein aggregation implicated in ALS progression. In May 2025, the company received United States Food and Drug Administration (FDA) approval for its investigational new drug (IND) application, and a phase 1 trial is planned to begin in Q3 2025.
In preclinical studies, Amisodin markedly preserved motor function and extended survival in the SOD1-G93A ALS mouse model, with no significant safety issues observed across pharmacological, genetic, and toxicological evaluations. PRG S&Tech has found that trimeric SOD1 exacerbates the effects of TDP-43 in ALS, and that Amisodin alleviates neurogeneration in a transgenic TDP-43 mouse model, suggesting broader disease-modifying potential.
PRG S&Tech’s phase 1 Amisodin studies are due to start later this year and will lay the groundwork for a phase 2 trial targeting both familial and sporadic ALS patients with SOD1 or TDP-43 pathology.
AutotacBio: targeted protein degradation
Dysregulation of autophagy, and a failure to degrade pathological proteins, such as tau, α-synuclein and TDP-43, is a hallmark of many neurodegenerative diseases. AutotacBio has developed the autophagy-targeting chimera (AUTOTAC) platform to go beyond merely inhibiting toxic proteins to eliminating them completely.
AUTOTAC is built around first-in-class, heterobifunctional small molecules that contain a ligand designed to bind to a protein of interest and another ligand that binds to the autophagy receptor p62, delivering the toxic protein for degradation.
Lead AUTOTAC candidates AB-12 and AB-19 target tau oligomers and aggregates in Alzheimer’s disease (AD) and progressive supranuclear palsy (PSP), respectively. While antibody- and inhibitor-based drugs targeting extracellular or intracellular species of tau have all failed over the past 20 years, AB-12/19 show remarkable degradation of intracellular tau neurofibrillary tangles (NFTs) in murine and canine models of tauopathic dementia and paralysis, accompanied by baseline rescue of cognitive, behavioral and neuromuscular functions. Both candidates have good absorption, distribution, metabolism, elimination and toxicity (ADMET)/drug metabolism and pharmaco-kinetics (DMPK) profiles and are currently in phase 1 trials in South Korea, with results expected by Q1 2026.
The preclinical AB-16 program, targeting α-synuclein oligomers and aggregates for disease-modification in synucleinopathies such as Parkinson’s disease, will be IND-ready soon.


