Nagase Viita is building on its rich history in saccharide innovation to improve antibody production. The company provides a stable and sustainable global supply of high-quality injectable-grade saccharides and invests in research and development (R&D) to find new ways to use the ingredients. Reflecting a focus on improving the well-being of people and the planet, Nagase Viita researchers have discovered a way to counter an emerging challenge in antibody production.
Founded as a starch syrup manufacturer in 1883, Nagase Viita established itself as a leading provider of ingredients across a range of industries by tackling unique and original research. Investment in R&D led to breakthroughs including the discovery of a method for mass-producing the disaccharide trehalose in 1994.
Today, Nagase Viita offers a portfolio of saccharide excipients under the SOLBIOTE brand. The portfolio features high-purity, low-endotoxin trehalose, sucrose and maltose products with applications in areas including the stabilization of protein-based pharmaceuticals.
Nagase Viita makes trehalose and maltose from starch using enzymes found in soil microorganisms. The company strictly controls the entire manufacturing process—from the procurement of raw materials to saccharide production and purification—at its factory in Japan. The manufacture of SOLBIOTE uses water, rather than International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q3C class 1, 2 or 3 solvents. The company’s approach ensures a reliable, sustainable supply of SOLBIOTE products.
Maintaining its history of innovation, Nagase Viita is continuing to uncover new uses for its saccharides in biopharmaceutical development. Recent breakthroughs include research that suggests trehalose could help antibody manufacturers counter a trend that has increased the cost and complexity of downstream purification.
The need for a new approach
The host cells that manufacturers use to express antibodies make both the target product and endogenous proteins. As upstream processing has advanced, antibody manufacturers have reported increased carry-over of host-cell-derived impurities into downstream processing.
This creates challenges. The endogenous host-cell proteins (HCPs) carried over are part of the harvested cell-culture fluid and must be separated from the antibody product. HCPs that remain part of the product can affect efficacy and cause immunogenic responses if administered to patients.
Manufacturers have traditionally used centrifugation and depth filtration to remove HCPs and host-cell DNA from harvested cell-culture fluid and meet their impurity targets. More recently, chromatographic clarification has emerged as a harvesting method that can effectively reduce carry-over impurities.
Researchers have found that the clearance of DNA using chromatography is less effective at high conductivity values1. Similarly, studies have shown that using buffer exchange to lower conductivity dramatically improves the removal of impurities in depth filtration2. These findings suggest companies can improve depth filtration and chromatographic clarification by cutting conductivity.
Researchers at Nagase Viita tested how additives used in biopharmaceuticals, such as amino acids and saccharides, affect conductivity values. Their studies found that some saccharides reduce conductivity. Sucrose and trehalose emerged as the leading candidates because they are widely used as cryoprotectants. Both saccharides protect cells and proteins from physical stress and, as such, have benefits in antibody production.
The researchers picked trehalose over sucrose for further testing because it is more stable at low pH and less likely to decompose, even if it remains in the elution step of immunoglobulin G-binding Protein A purification. Prior to the study, there was a lack of evidence about the effect of trehalose on antibody production. To fill the knowledge gap, the researchers added trehalose after the cultivation of Chinese hamster ovary (CHO) cells (Fig. 1).
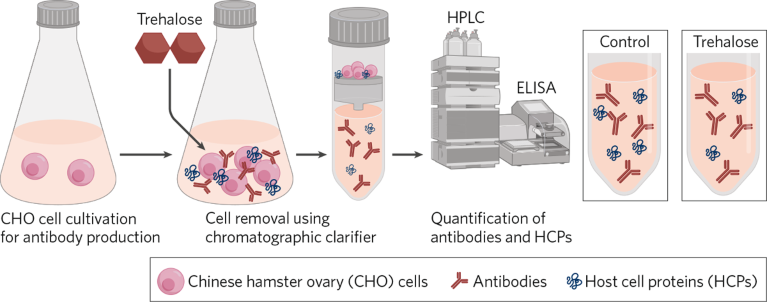
Fig. 1 | Improving antibody harvesting with trehalose. Adding trehalose after cultivation of CHO cells, culture fluid was clarified by a chromatographic clarifier device (3M Harvest RC). The addition of trehalose reduced host-cell proteins (HCPs) by approximately 20% with no effect of antibody yield. ELISA, enzyme-linked immunosorbent assay; HPLC, high-performance liquid chromatography.
How trehalose enhances processing
The research suggests using trehalose could be an entirely new approach to reducing carry-over of impurities. Adding trehalose to the diatomaceous earth (DE) filtration process cut levels of HCPs and host cell DNA in the filtrates. Trehalose had no effect on antibody concentrations. The impurities in unfiltered samples, mixed with CHO cells, DE and trehalose, were also reduced. This confirmed the known cytoprotective effects of trehalose.
Additional data suggested trehalose does more than just protect cells. Adding trehalose after the removal of CHO cells using centrifugation lowered lactate dehydrogenase levels in the filtrate. The result suggested trehalose enhances adsorption of the impurities to the membrane.
The researchers also found trehalose enhances chromatographic clarification. After cultivating CHO cells for seven days, the researchers mixed the culture fluid with fresh medium containing trehalose and used a 3M Harvest RC Chromatographic Clarifier to clarify the mixture.
Adding trehalose reduced HCP levels in the filtrate after chromatographic clarification. The effect on HCP levels was dose dependent. Using 200 mM of trehalose, the researchers saw HCP reductions of 18.1% to 25.9% compared to controls. There was no effect on antibody concentrations. The study suggested using 100–200 mM of trehalose can reduce HCP levels by 10% to 30%.
The reductions in HCP carry-over have implications for the purification process. Researchers have reported that fouling due to feed material has the most impact on lifetime of the Protein A resin and performance of the purification3. By lowering HCP levels, manufacturers could extend the life of the Protein A resin used in downstream chromatography processes or simplify purification processes.
Nagase Viita looked at Protein A chromatography in the study. Filtrates obtained from chromatographic clarification with 200 mM of trehalose were purified using Protein A chromatography. The researchers found the antibody concentrations in lots clarified with trehalose were 6.8–7.5% higher than the controls. HCP levels in the trehalose lots were 8.7–16.8% lower than in the controls.
Size-exclusion chromatography analysis performed after purification showed adding trehalose had no adverse effects, such as an increased high-molecular-weight aggregates. The analysis confirmed that trehalose helps lower impurities without causing unwanted aggregation and compromising the quality of the final antibody product.
There are two potential explanations for the effects of trehalose. First, the excipient is known to have cytoprotective effects. Protecting host cells from physical stress during filtration could reduce the levels of impurities that leak from the cells and need removing from the mixture. Second, the study showed trehalose affects conductivity, a variable that determines the effectiveness of impurity removal. Adding 10% of trehalose crystal to the culture supernatant reduced conductivity by approximately 1.6 mS/cm (Fig. 2).
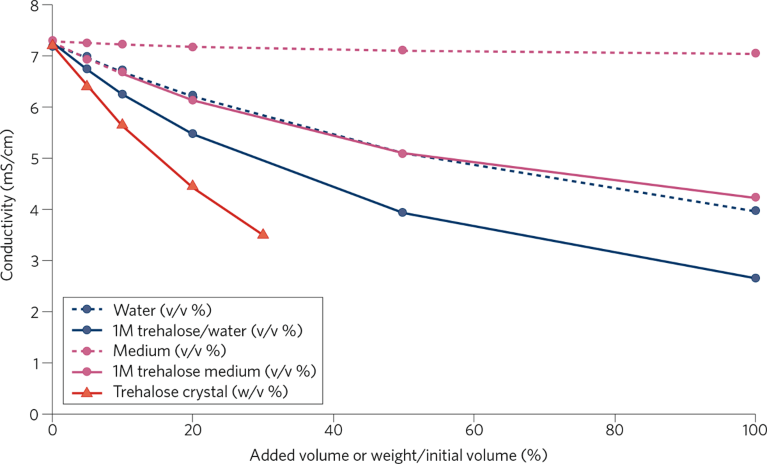
Fig. 2 | Decrease in conductivity of culture supernatant by addition of trehalose. 1M of trehalose in water or medium and trehalose crystal were added to the culture supernatant. After thorough mixing, the conductivity was measured.
Researchers have linked lower conductivity to improved filtration, suggesting that the phenomenon may be a factor in the effect of trehalose on HCP levels. Further research is needed to understand why the level of supernatant conductivity affects the effectiveness of filtration. One hypothesis is that low conductivity enhances molecular interactions, increasing HCP adsorption.
Supporting people and the planet
The trehalose research advances Nagase Viita’s mission to harness the power of nature to achieve accessible healthcare and sustainability in the industry. The company uses microorganisms and enzymes to create a diverse range of materials that support the well-being of people and the planet.
EcoVadis, one of the world’s most trusted providers of business sustainability ratings for global supply chains, has recognized the success of Nagase Viita’s environmental efforts. The company received the highest ‘Platinum’ rating in EcoVadis’ sustainability survey two years in a row, most recently in January 2025. The Platinum rating is reserved for businesses that achieve scores in the top 1% of all eligible companies, showing that Nagase Viita is at the forefront of global industrial efforts to make supply chains more sustainable.
Nagase Viita confirms with suppliers that raw materials are sourced without causing deforestation or human rights violations, promotes energy conservation, and uses inventory control and manufacturing planning to reduce waste. In addition, to reduce environmental impact, the company strives to decrease carbon emissions during procurement and production processes, minimize post-manufacturing waste and promote wastewater treatment.
The SOLBIOTE portfolio aims to provide sustainable solutions for biopharmaceuticals. The trehalose study is testament to the company’s push to enhance well-being by improving the quality of biopharmaceuticals. Using trehalose to improve impurity removal early in the process can reduce the burden on, and need for, expensive downstream-purification steps. The benefits could be significant.
Trehalose enhances chromatographic clarification, enabling manufacturers to switch to simpler, cheaper processes or extend the life of their Protein A resin. Companies that incorporate the excipient into their processes could also increase yields in Protein A purification.
The research shows that using trehalose could be an entirely new approach to reducing the carry-over of impurities into later purification processes. With carry-over impurities increasing in recent years, the new approach may empower companies to overcome a significant, intensifying challenge and ensure the cost-effective, sustainable production of antibodies that improve the lives and well-being of patients.


