The Beijing-based biotechnology company CorreGene was co-founded by Peking University alumni in 2016 to develop innovative T-cell receptor (TCR) therapies. CorreGene’s T-cell engager (TCE) pipeline comprises multiple agents that can simultaneously bind to T cells through a T-cell surface glycoprotein cluster of differentiation 3 (CD3) antibody-binding domain, and to tumor cells through a TCR-fusion protein that can recognize intracellular tumor-associated antigens (TAAs) presented by peptide–major histocompatibility complex (pMHC) molecules.
This TCR–TCE approach grants access to the >90% of TAAs that are inaccessible to conventional membrane-targeting antibody therapeutics. “The expanded druggable space allows us to target numerous TAAs exhibiting exceptional tumor specificity and widespread expression across various tumors, thereby mitigating the on-target off-tumor toxicity that has hindered the progression of TCEs for solid tumor applications,” said Xingwang Xie, CorreGene’s founder/chairman and CEO. Moreover, TCR-bispecific agents can drive more durable anti-tumor responses by engaging with pMHC on dendritic cells, thus promoting antigen spreading and enhancing immune activation1 (Fig. 1).
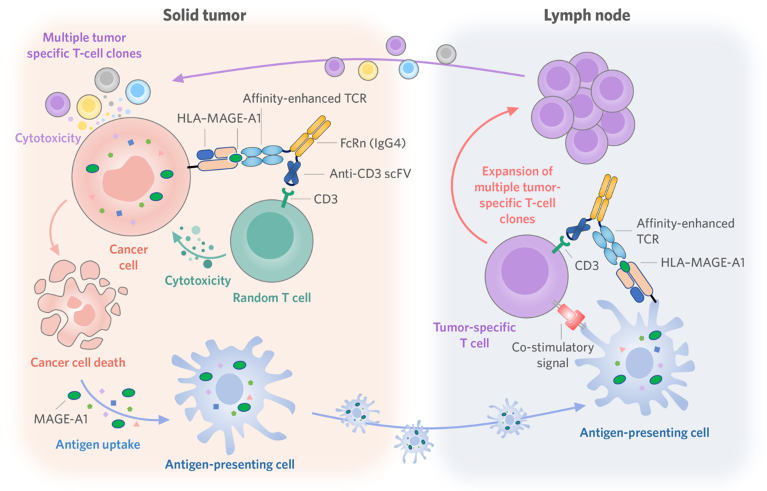
Fig. 1 | CorreGene’s T-cell engager, CRPA1A2, targets the tumor-associated melanoma-associated antigen 1 (MAGE-A1) presented by cancer cells and dendritic cells to trigger a specific and durable anti-tumor response. CD3, cluster of differentiation 3; FcRn, IgG receptor FcRn large subunit p51; HLA, human leukocyte antigen; IgG4, immunoglobulin G4; scFV, single-chain variable fragment; TCR, T-cell receptor.
The advantages of targeting pMHC complexes have sparked interest in developing TCR-mimic antibodies. “These antibodies often display a ‘hot-spot’ binding pattern, leading to increased cross-reactivity compared to affinity-enhanced natural TCRs,” Xie explained. In contrast, natural TCRs leverage a broader, more evenly distributed energetic footprint to interact with pMHC molecules, resulting in superior specificity2.
CorreGene’s lead asset, CRPA1A2, is the first TCR–TCE to target the cancer/testis antigen melanoma-associated antigen 1 (MAGE-A1) presented by the human leukocyte antigen (HLA) genotype A*02:01 found in 48% of Caucasians and 23% of Chinese populations. Preclinical studies have demonstrated the drug’s exceptional specificity and potency against tumor cells3. In mouse models of cancer, treatment with CRPA1A2 led to robust T-cell tumor infiltration, marked tumor regression and only transient, mild cytokine release. There were no signs of organ toxicity or weight loss, indicating a reduced risk of cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome compared with other antibody-based TCEs or T-cell therapeutics.
CorreGene is filing an investigational new drug (IND) application to the US Food and Drug Administration (FDA), with preliminary multinational phase 1 clinical data expected in 2026. The study, focusing on patients with advanced solid tumors lacking standard of care, is set to commence in Australia by the end of this year.
MAGE-A1 is expressed in approximately 50% of lung squamous cell carcinomas, 55% of liver hepatocellular carcinomas, 32% of esophageal carcinomas and 18% of stomach adenocarcinomas, but is virtually undetectable in normal adult tissues4. “The high specificity and broad expression across solid tumors make MAGE-A1 an ideal therapeutic target with enormous potential for multiple solid tumors,” Xie noted.
Developing next-generation TCEs
CorreGene’s solid technology platforms support the cloning of functional, soluble TCRs and affinity optimization to achieve potent anti-tumor effects.
The company’s SMART-TCR affinity maturation platform uses T-cell display technology that enables high-throughput, single-cell resolution, function-based screening to identify the most effective candidate TCRs for TCE drug development. “A fundamental advantage of our platform is that we can screen candidate TCRs based on their antigen-specific activity, rather than relying solely on binding affinity,” said Xie. In addition, stringent screening against a comprehensive panel of human whole-genome-derived antigens eliminates off-target TCRs that could cross-react with healthy tissues, ensuring the progression of safe candidates.
With the CorEngager Platform, CorreGene can adapt native insoluble TCRs to produce soluble TCE protein drugs that exhibit exceptional pharmacokinetics (with a half-life of over 14 days), tumor penetration, and chemistry, manufacturing, and controls (CMC) features. CorEngager molecules can be produced at yields exceeding 5 g/L, facilitating consistent, large-scale production and supporting broad clinical and commercial deployment. “The CorEngager platform offers a robust solution to the longstanding tech-nical challenges of expressing transmembrane TCR molecules in a soluble, functional form,” Xie added.
CorreGene is interested in establishing global partnerships to advance TCE therapies to the clinic and to leverage its platform technologies to develop new TCR-based drug modalities, including TCR conjugates and TCR-T-cell therapies. By redirecting T cells against tumor cells, while minimizing off-tumor toxicity and cytokine release, CorreGene is committed to delivering transformative off-the-shelf cancer therapies.


