Since insulin’s discovery in the 1920s, science has delivered nine classes of drugs to treat type 2 diabetes (T2D). These have improved life for many; but more than 100 years later, the disease remains widespread and progressively degenerative. An estimated 828 million adults were affected globally in 2022, up by 630 million since 1990, according to research from the NCD Risk Factor Collaboration (NCD-RisC)1.
The complications of T2D are severe, from blindness and organ failure to heightened infection risk. As a result, annual T2D-related healthcare costs reached $966 billion in 2021 and are projected to exceed $1 trillion by 2045, based on the International Diabetes Federation’s estimates.
By shifting the therapeutic focus from symptom control to disease modification, and thereby reducing complications, Hua Medicine’s development of the world’s first approved glucokinase activator could mark a historic breakthrough in the treatment of T2D.
The history of Hua Medicine
Headquartered in Shanghai, China, and founded by CEO and executive director Li Chen in 2011, Hua Medicine focuses on innovative and potentially disease-modifying therapeutics for T2D based on glucose homeostasis research. The company’s phase 3 monotherapy (SEED) and remission (DREAM) studies of its flagship drug, dorzagliatin, suggest that it could change the treatment paradigm by restoring the body’s ability to sense and regulate blood glucose levels, as well as preventing the ongoing loss of β-cell function.
Supported by international blue-chip investors, including ARCH Venture Partners, Venrock, Fidelity Investments and WuXi AppTec, Hua Medicine’s leadership team includes world-class scientists and industry veterans. The main goal of the company is to advance the treatment of T2D by addressing the root cause of the disease, rather than targeting high blood-sugar levels, a well-recognized primary symptom of the disease.
Glucokinase activators bringing new hope to type 2 diabetes patients
Hua Medicine’s research is primarily based on the role of glucokinase in maintaining glucose homeostasis. The research stems from the work of German-born researcher Franz Matschinsky at Washington University in Saint Louis and at the Penn Rodebaugh Diabetes Center of the University of Pennsylvania Medical School. Matschinsky showed that glucokinase, an enzyme also known as hexokinase IV, acts as a glucose sensor and controls insulin secretion in pancreatic β-cells. This process allows the body to autonomously maintain glucose levels within a healthy range. Matschinsky’s work formed the basis of the development of a number of glucokinase activators, but progress has been challenging, with some projects halted because of unwanted side effects and/or insufficient efficacy2. In 2011, Hua Medicine in-licensed Roche’s program of glucokinase activators, including dorzagliatin.
Dorzagliatin (HuaTangNing; 华堂宁) is a global first-in-class, dual-acting, allosteric glucokinase full activator that targets both pancreatic and hepatic glucokinases. In preclinical studies, dorzagliatin regulated glucagon-like peptide 1 receptor (GLP-1) secretion, improved glucose-stimulated insulin secretion, repaired islet function and improved cognitive impairment (Fig. 1).
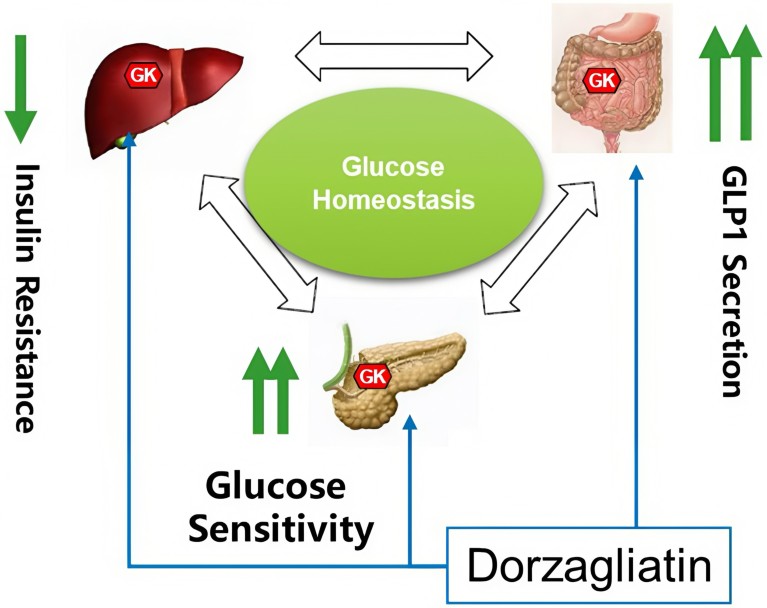
Fig. 1 | A differentiated first-in-class antidiabetic drug advancing diabetes care globally. Glucokinase (GK) is an enzyme that functions as a glucose sensor and plays a central role in glucose homeostasis. Impairment or loss of GK sensor function leads to impaired glucose sensitivity, insulin resistance and eventually diabetes. By repairing GK sensor function, dorzagliatin was observed to have improved β-cell function and glucose sensitivity, reduced insulin resistance and increased GLP-1 secretion in type 2 diabetes patients in clinical trials6.
Entering clinical trials in 2013, a phase 2 study published in 2018 showed significant and dose-dependent reductions in glycated hemoglobin (HbA1c) in Chinese patients with T2D3. Further studies showed that these reductions can be sustained, including during a 52-week trial period4. The drug is well tolerated with only mild hypoglycemia. Following phase 3 trials, dorzagliatin was approved in China in 2022 for the twice-daily oral treatment of T2D alone or in combination with metformin. Also approved in China for the treatment of diabetic kidney disease (DKD), dorzagliatin is one of only a few oral T2D medications approved for all stages of DKD without dose adjustment. Dorzagliatin was included in China’s National Reimbursement Drug List effective as of 1 January 2024, significantly expanding accessibility and affordability of the medication nationwide.
Initially marketed by Bayer Healthcare, Hua Medicine assumed full commercialization responsibility for dorzagliatin in January 2025. To date, it has been prescribed to over 200,000 patients in around 2,700 hospitals, validating the company’s approach of targeting and restoring the function of the glucokinase enzyme.
Moving from symptom control to remission
Remission in diabetes is generally defined as maintaining blood glucose within the normal range (HbA1c <48 mmol/mol or 6.5% for at least three months), tracked by glucose time in range (TIR). In studies of intensive lifestyle management or significant weight loss (for example, bariatric surgery or very low-calorie diets), remission can substantially reduce the risk of chronic kidney disease and cardiovascular disorders, but maintaining these changes can be a challenge. That is why a drug that can put T2D into prolonged remission with no side-effects is of enormous value (Fig. 2).
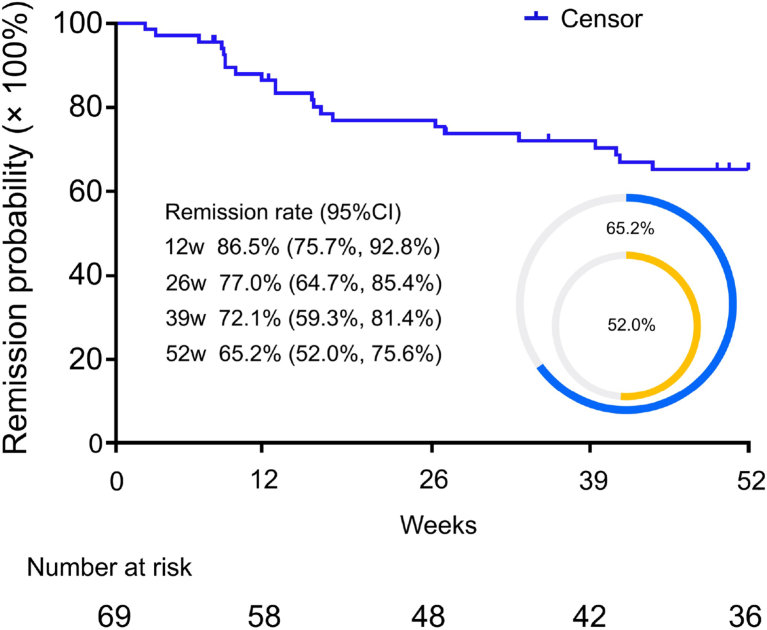
Fig. 2 | The DREAM investigator-initiated study related to diabetes remission5. The remission rate was 65.2% (95% CI: 52.0%, 75.6% in blue) at week 52 based on the judgement by the investigators. The DREAM study was initiated before American Diabetes Association (ADA) type 2 diabetes (T2D) remission consensus was released in Aug 2021. A post hoc analysis using ADA definition of remission (HbA1c<6.5% measured at least 3 months after discontinuation of treatment) was also conducted. The survival analysis showed that the remission rate at week 12 was 52.0% (95% CI: 31.2%, 69.2% in yellow). The primary endpoint was diabetes remission rate at week 52, and was estimated by the Kaplan-Meier method.
Existing data suggest that dorzagliatin does just that. The company’s 52-week placebo-controlled phase 3 SEED trial evaluated dorzagliatin as a monotherapy in 463 newly diagnosed and treatment-naive patients with T2D. Placebo-treated patients switched to the active drug at 24 weeks and continued until week 52. At week 24, there were statistically significant reductions in HbA1c and better glycemic control in the dorzagliatin group, with no severe hypoglycemia events or drug-related serious events4. To understand dorzagliatin’s impact on long-term remission, several independent investigators from the SEED trial initiated the DREAM study. This was a 52-week post-treatment observational study that followed 69 participants from SEED who had achieved stable glycemic control and discontinued all antidiabetic medication after the SEED study’s conclusion. At the end of DREAM’s 52-week drug-free observation period, a 65% diabetes remission rate (applying the Kaplan–Meier methodology) was observed5, suggesting that dorzagliatin may be disease-modifying (Fig. 2).
Real-world data are valuable, both for regulators and for healthcare professionals making choices about treatment options. Accordingly, a large post-marketing real-world multicenter study (BLOOM) is underway in 80 hospitals in China. The BLOOM study has enrolled 2,000 patients with T2D at different stages, including people who are elderly, obese, or have multiple diabetic complications. In addition to observing safety for one year, the study will assess the efficacy of dorzagliatin in combination with multiple glucose-lowering drugs such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors, GLP-1 receptor agonists, insulin and dipeptidyl peptidase 4 (DPP-4) inhibitors. The study is enrolled and 80% of the patients have completed six months of observation. Another single-center real-world study (ASPIRE-D) has enrolled 300 T2D patients in China, with interim results presented at the 2025 American Diabetes Association (ADA)’s scientific sessions. More than 70% of the patients in the ASPIRE-D study received three or more glucose-lowering drugs (including dorzagliatin). The interim analysis confirmed clinically meaningful reductions in HbA1c levels, improved continuous glucose-monitoring metrics, and enhanced β-cell function.
Combination therapy is an important approach for individuals whose diabetes isn’t managed sufficiently with a first-line treatment. In US phase 1 trials of dorzagliatin in combination with the DPP-4 inhibitor sitagliptin or the SGLT-2 inhibitor canagliflozin, there were no drug interactions, and the combinations showed synergies in glycemic control and improvement of β-cell function. In the DAWN double-blind, placebo-controlled phase 3 study, patients unable to control their diabetes on metformin alone were given metformin with either placebo or dorzagliatin for 24 weeks and then switched to open label metformin/dorzagliatin for 28 weeks. The combination improved glycemic control with an acceptable safety profile.
In the Hong Kong-based SENSITIZE study involving people with glucokinase maturity-onset diabetes of the young (GCK-MODY) or recent-onset T2D, dorzagliatin at 25 mg and 50 mg improved insulin secretion rates and β-cell glucose sensitivity, supporting the drug’s potential to tackle the glucose-sensing issues underlying T2D. The ongoing study will evaluate the role of dorzagliatin in preventing the development of T2D in prediabetic individuals.
Building for the future
Dorzagliatin plays an important role in China, where the incidence of T2D is rising fast. NCD-RisC estimated that there were 148 million cases in 2022, the largest proportion worldwide, with around 78 million cases untreated. This is driven by several factors, including a traditional diet based on rice and noodles and an increasingly Western-influenced lifestyle.
Dorzagliatin’s relatively low cost, its potential as a disease-modifying agent to induce remission in newly diagnosed and existing patients (alone and in combination), and its approval in people with DKD suggests applications in a wider global market. Hua Medicine is already pursuing markets in Hong Kong and Macau. However, beyond these two territories that accept China’s National Medical Products Administration approval, Hua Medicine has come up against barriers. International regulators such as those in the United States, Europe and Japan do not accept data from pivotal studies conducted solely in China, and additional trials would be costly.
At the same time, Hua Medicine is developing a second-generation dorzagliatin prodrug with potential in international markets. HM-002-1005 is a once-daily oral formulation in development for diabetes, obesity, DKD, frailty and sarcopenia. HM-002-1005 is a new chemical entity (NCE), and the company holds patents into the 2040s.
As a first step towards development and commercialization outside of mainland China, Hua Medicine completed a phase 1a trial of HM-002-1005 in the United States in 2024. The randomized, double-blinded, placebo-controlled, single ascending dose study in 40 subjects with T2D confirmed that once daily HM-002-1005 rapidly converted to the parent drug, with comparable pharmacokinetics and pharmaco-dynamics to currently approved twice-daily dorzagliatin. A phase 1b multiple ascending dose trial in the United States is planned for late 2025 or early 2026.
Hua Medicine plans to seek an international partner for further development and commercialization of its glucokinase program.


