During infection, the human immunodeficiency virus (HIV) inserts itself into the host genome as a chromosomally-integrated provirus that persists in infected cells. Although antiretroviral therapies can contain HIV in the long term by preventing the retrovirus from replicating and causing acquired immunodeficiency syndrome (AIDS), they do not target the provirus itself and therefore cannot eliminate the infection. A promising way to cure HIV is to inactivate or remove the integrated HIV provirus using currently available gene-editing tools such as clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR-associated protein 9 (Cas9). Unfortunately, although these can be used to eliminate key genes, like the provirus, they cleave the double-stranded DNA helix and rely on a cell’s natural repair mechanisms to restore or mend DNA alterations—a process that research shows can cause errors with unpredictable efficiencies and consequences.
PROVIREX, a privately held biotech company based in Hamburg, Germany, has an innovative way of overcoming these issues. The company has developed an error-free genome-editing technology that precisely cuts out proviruses, enabling life-threatening persistent viral infections to be eradicated. Phase 1b/2a clinical trials of the company’s precision technology in people living with HIV are scheduled to start later this year.
Recombinase-based genome surgery
With its initial focus on HIV, the research team behind PROVIREX first generated an HIV-specific recombinase using direct-evolution techniques and showed that it recognizes and targets a portion of the long terminal repeats (LTRs) that flank the HIV provirus DNA. The team demonstrated that the recombinase had a considerable antiviral effect in humanized mice infected with human HIV. The researchers then developed a designer recombinase that specifically recombines a very conservative and clinically highly relevant HIV LTR sequence1. This recombinase called Brec1—a proprietary PROVIREX protein—specifically and efficiently cuts out the provirus of more than 90% of all known HIV strains and subtypes.
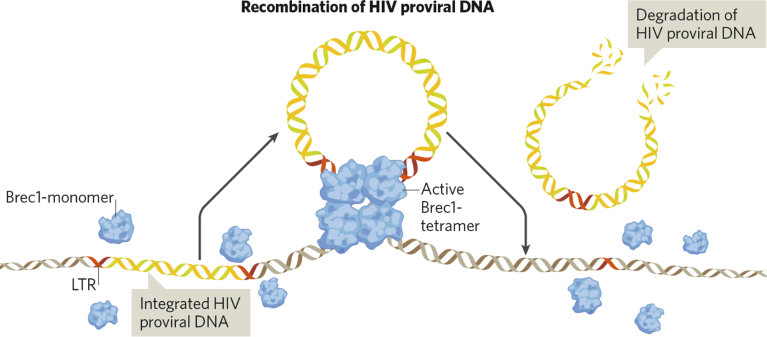
PROVIREX’s error-free genome-editing precision technology. Precisely cutting out proviruses to halt infection.
“The major advantage of the Brec1 recombinase is that the cutting and mending of the DNA is accomplished independently of further helper molecules (like guide RNAs) or the host DNA-repair system,” explained Frank Buchholz, PROVIREX co-founder, and professor of medical systems biology and dean of research at the Medical Faculty Carl Gustav Carus of the Technical University of Dresden. “This is a much safer technology because, in contrast to all other gene-editing approaches, Brec1 acts with nucleotide precision and reassembles the genome without errors.”
Indeed, preclinical studies demonstrate that Brec1 excises the HIV-1 provirus with high specificity from the genome of infected host cells with unparalleled activity and lasting antiviral effects in both in vitro and in vivo models, including mice humanized with patient-derived cells. In these studies, plasma viral load declined to levels below the detection limits of the most-sensitive assay systems and without viral rebound or unwanted side effects. The Brec1 technology is entering clinical testing in 2025 in a phase 1b/2a clinical trial (HIVCure) at the University Medical Center Hamburg-Eppendorf in collaboration with the Leibniz Institute of Virology (from which PROVIREX was spun-out in 2019).
Currently, delivery of the Brec1 recombinase involves transfecting the Brec1 gene into peripheral blood hematopoietic stem cells, which are then transplanted back into the patient. To facilitate a broad and convenient global application of the Brec1 technology in people living with HIV in the future, PROVIREX is also developing an advanced direct-delivery technology to efficiently guide encapsulated recombinase to the target cells in vivo.
Partnering to progress potential cures
Although the primary focus of PROVIREX is currently on curing HIV, its pipeline also contains a recombinase that targets infection with the human T-lymphotropic virus (HTLV) frequently causing blood cancer. Moreover, because of its versatility and effectiveness, the technology has great potential for additional applications, such as curing other persistent viruses or treating hereditary diseases.
Having raised Series A funding in 2022, PROVIREX is interested in establishing a collaboration to further develop its in vivo delivery technology and, further down the line, the company would be interested in partnering with a biotechnology or pharmaceutical organization to help progress Brec1 through later-stage clinical trials. “Our vision is to improve the lives of people living with HIV and other life-threatening persistent infections,” said PROVIREX co-founder and CSO Jan Chemnitz. “Using our recombinase-based genome-surgery technology to precisely cut out integrated HIV from infected cells, we are offering a chance to permanently control the infection and potentially cure people living with HIV.”


