Antibody–drug conjugates (ADCs) deliver potent anti-cancer drugs directly to tumor cells by taking advantage of the ability of antibodies to recognize cancer cell receptors. This targeted approach aims to minimize damage to healthy tissues, reducing the side effects commonly associated with traditional chemotherapy, and enhancing the effectiveness of treatment.
Despite their potential, the development of ADCs has not been straightforward. “There has been quite a lot of trial and error over the years, it has taken a long time to figure out a way of putting the antibody, the linker and the payload together to achieve a better therapeutic index than chemotherapeutics,” said Michael Torres, CEO and co-founder of the Texas-based biotechnology company CrossBridge Bio.
Recent advances in ADC design have led to a rapid increase in the number of approved agents for hematological cancers and solid tumors. Yet, as Torres explained, there are still important challenges that need to be overcome, such as instability and deconjugation of the payload before it reaches its target, which can cause severe side effects.
CrossBridge Bio is leveraging technology from The University of Texas Health Science Center at Houston (UTHealth Houston) to create more stable ADCs that can deliver multiple payloads. With a grant from the Cancer Prevention and Research Institute of Texas (CPRIT), CrossBridge Bio will advance its lead candidate, the dual-payload ADC CBB-120 for the treatment of trophoblast cell-surface antigen 2 (TROP-2)-positive solid tumors, to investigational new drug (IND)-enabling studies. Safety testing in non-human primates is expected to start in 2025.
Harnessing new technology to produce next-generation ADCs
Torres was working as an entrepreneur in residence at the Texas Medical Center Innovation’s Accelerator for Cancer Therapeutics—a CPRIT-funded program to help faculty launch companies—when he came across Zhiqiang An and Kyoji Tsuchikama’s pioneering work on therapeutic antibodies and linker technologies1,2.
Torres soon realized the potential of their joint expertise to drive a step change in ADC efficacy and safety, so he approached them to co-found CrossBridge Bio in 2023. “We can now put the pieces together, take the technology out of the academic institute and achieve really important milestones,” he said.
CrossBridge Bio uses a proprietary linker that is stable against proteases and is highly hydrophilic, preventing premature payload release and potential aggregation. Linkers can be conjugated to specific antibody sites and loaded with more than one drug, so they are better equipped to combat cancer predisposition, drug resistance and tumor heterogeneity.
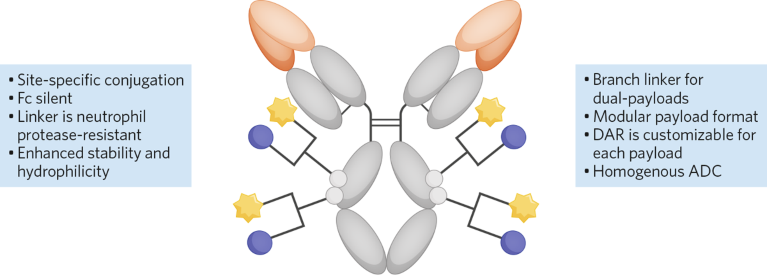
Qualities of CrossBridge Bio's dual-payload ADCs. ADC, antibody–drug conjugate; DAR, drug–antibody ratio.
Historically, ADCs with dual payloads have been very difficult to manufacture, but CrossBridge Bio is getting good yields using click chemistry to reliably and specifically conjugate different payloads, minimizing waste and side reactions. “Our click-chemistry-based platform allows for modular assembly of readily available linker and payload molecules, so we can quickly create dual-payload ADC libraries and identify optimal ADC designs,” Torres said.
With its innovative linker technology, CrossBridge Bio can deliver interesting drug combinations stably to the site of action to potentially achieve synergistic effects. “As CBB-120 is tested on a variety of tumor types and indications and we de-risk the technology, it will be fairly easy to push ahead with other targets and payloads,” he added.
Growing the pipeline
Driven by advances in tumor cell biology and personalized medicine, as well as input from key opinion leaders in the field, CrossBridge Bio is exploring the breadth of molecules that can be conjugated, and the efficacy and safety of different payload combinations. Torres and Dan Pereira, CrossBridge Bio’s CSO, have regular conversations with highly regarded clinicians working at renowned cancer institutes, such as MD Anderson and the Dana-Farber Cancer Institute, and pharmaceutical companies. “Their insights are extremely valuable for prioritizing pipeline candidates,” Pereira said.
Pereira joined the company in March 2024 and brings a wealth of experience in advancing antibody-based cancer therapeutics into clinical trials. He contributed to the development of five US Food and Drug Administration (FDA)-approved antibodies for oncology; the most recent being Padcev (enfortumab vedotin) for the treatment of urothelial cancer. He is very enthusiastic about CrossBridge’s pipeline: “We are following up CBB-120 with other agents that have first-in-class as well as best-in-class potential.”
CrossBridge Bio is focusing on agents with a highly differentiated ADC make-up for cancers of unmet need such as colorectal, ovarian, prostate and non-small-cell lung cancer (NSCLC). “At the moment, what really differentiates us in the ADC world is multi-payload, but we are also thinking of coupling our technology with bispecific antibodies to better tackle tumors and other diseases that would benefit from a dual-targeting approach, such as immune or inflammatory diseases,” he added.
CrossBridge Bio plans to actively pursue strategic partnerships and collaborations as it advances its therapeutic candidates through preclinical development. By engaging in partnerships and deal-making, CrossBridge Bio aims to expand its pipeline, increase its market reach and bring transformative therapies to patients more rapidly.


