Abstract
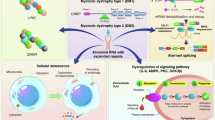
Dystrophia myotonia type 1 (DM1; Steinert's disease; myotonic dystrophy) is an autosomal dominant disorder due to a large CTG expansion in the 3′-untranslated region (UTR) of the DM protein kinase (DMPK) gene. Transcription of this gene yields a long CUGn-containing mutant (mut) RNA, in which clinical disease is associated with repeats of n=100–5000. Phenomenologically, the expression of mut RNA is correlated with the morphologic observation of ribonucleoprotein precipitates (‘foci’) in the nuclei of DMPK-expressing cells. The prevailing view is that the identification of proteins in these foci is the sine qua non of protein–mut RNA interactions. In this viewpoint, I contend that this is an unwarranted inference that falls short in explaining published data. A new model of mut RNA–protein interactions is proposed with distinct binding properties for soluble and insoluble (focus) mut RNA that accommodate these data without exclusions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hamshere MG, Newman EE, Alwazzan M, Athwal BS, Brook JD : Transcriptional abnormality in myotonic dystrophy affects DMPK but not neighboring genes. Proc Natl Acad Sci USA 1997; 94: 7394–7399.
Davis BM, McCurrach ME, Taneja KL, Singer RH, Housman DE : Expansion of a CUG trinucleotide repeat in the 3′ untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc Natl Acad Sci USA 1997; 94: 7388–7393.
Klesert TR, Cho DH, Clark JI et al: Mice deficient in Six5 develop cataracts: implications for myotonic dystrophy. Nat Genet 2000; 25: 105–109.
Sarkar PS, Appukuttan B, Han J et al: Heterozygous loss of Six5 in mice is sufficient to cause ocular cataracts. Nat Genet 2000; 25: 110–114.
Timchenko LT, Miller JW, Timchenko NA et al: Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res 1996; 24: 4407–4414.
Timchenko LT, Timchenko NA, Caskey CT, Roberts R : Novel proteins with binding specificity for DNA CTG repeats and RNA CUG repeats: implications for myotonic dystrophy. Hum Mol Genet 1996; 5: 115–121.
Philips AV, Timchenko LT, Cooper TA : Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 1998; 280: 737–741.
Timchenko NA, Cai ZJ, Welm AL, Reddy S, Ashizawa T, Timchenko LT : RNA CUG repeats sequester CUGBP1 and alter protein levels and activity of CUGBP1. J Biol Chem 2001; 276: 7820–7826.
Mankodi A, Takahashi MP, Jiang H et al: Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol Cell 2002; 10: 35–44.
Charlet-B N, Savkur RS, Singh G, Philips AV, Grice EA, Cooper TA : Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol Cell 2002; 10: 45–53.
Ho TH, Bundman D, Armstrong DL, Cooper TA : Transgenic mice expressing CUG-BP1 reproduce splicing mis-regulation observed in myotonic dystrophy. Hum Mol Genet 2005; 14: 1539–1547.
Miller JW, Urbinati CR, Teng-Umnuay P et al: Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J 2000; 19: 4439–4448.
Kanadia RN, Johnstone KA, Mankodi A et al: A muscleblind knockout model for myotonic dystrophy. Science 2003; 302: 1978–1980.
Dansithong W, Paul S, Comai L, Reddy S : MBNL1 is the primary determinant of focus formation and aberrant insulin receptor splicing in DM1. J Biol Chem 2005; 280: 5773–5780.
Ebralidze A, Wang Y, Petkova V, Ebralidse K, Junghans RP : RNA leaching of transcription factors disrupts transcription in myotonic dystrophy. Science 2004; 303: 383–387.
Taneja KL, McCurrach M, Schalling M, Housman D, Singer RH : Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J Cell Biol 1995; 128: 995–1002.
Mankodi A, Urbinati CR, Yuan QP et al: Muscleblind localizes to nuclear foci of aberrant RNA in myotonic dystrophy types 1 and 2. Hum Mol Genet 2001; 10: 2165–2170.
Houseley JM, Wang Z, Borck GJR et al: Myotonic dystrophy associated expanded CUG repeat muscleblind positive ribonuclear foci are not toxic to Drosophila. Hum Mol Genet 2005; 14: 873–883.
Fardaei M, Larkin K, Brook JD, Hamshere MG : In vivo co-localisation of MBNL protein with DMPK expanded-repeat transcripts. Nucleic Acids Res 2001; 29: 2766–2771.
Fardaei M, Rogers MT, Thorpe HM et al: Three proteins, MBNL, MBLL and MBXL, co-localize in vivo with nuclear foci of expanded-repeat transcripts in DM1 and DM2 cells. Hum Mol Genet 2002; 11: 805–814.
Mankodi A, Teng-Umnuay P, Krym M, Henderson D, Swanson M, Thornton CA : Ribonuclear inclusions in skeletal muscle in myotonic dystrophy types 1 and 2. Ann Neurol 2003; 54: 760–768.
Jiang H, Mankodi A, Swanson MS, Moxley RT, Thornton CA : Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Hum Mol Genet 2004; 13: 3079–3088.
de Haro M, Al-Ramahi I, De Gouyon B et al: MBNL1 and CUGBP1 modify expanded CUG-induced toxicity in a Drosophila model of myotonic dystrophy type 1. Hum Mol Genet 2006; 15: 2138–2145.
Ho TH, Savkur RS, Poulos MG, Mancini MA, Swanson MS, Cooper TA : Colocalization of muscleblind with RNA foci is separable from mis-regulation of alternative splicing in myotonic dystrophy. J Cell Sci 2005; 118: 2923–2933.
Mahadevan MS, Yadava RS, Yu Q et al: Reversible model of RNA toxicity and cardiac conduction defects in myotonic dystrophy. Nat Genet 2006; 38: 1066–1070.
Yuan Y, Compton SA, Sobczak K et al: Muscleblind-like 1 interacts with RNA hairpins in splicing target and pathogenic RNAs. Nucleic Acids Res 2007; 35: 5474–5486.
Timchenko LT, Iakova P, Welm AL, Cai ZJ, Timchenko NA : Calreticulin interacts with C/EBPalpha and C/EBPbeta mRNAs and represses translation of C/EBP proteins. Mol Cell Biol 2002; 22: 7242–7257.
Mili S, Steitz JA : Evidence for reassociation of RNA-binding proteins after cell lysis: implications for the interpretation of immunoprecipitation analyses. RNA 2004; 10: 1692–1694.
Savkur RS, Philips AV, Cooper TA : Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat Genet 2001; 29: 40–47.
Junghans RP, Waldmann TA : Metabolism of Tac (IL2Rα): physiology of cell surface shedding and renal elimination, and suppression of catabolism by antibody binding. J Exp Med 1996; 183: 1587–1602.
Michalowski S, Miller JW, Urbinati CR, Paliouras M, Swanson MS, Griffith J : Visualization of double-stranded RNAs from the myotonic dystrophy protein kinase gene and interactions with CUG-binding protein. Nucleic Acids Res 1999; 27: 3534–3542.
Kino Y, Mori C, Oma Y, Takeshita Y, Sasagawa N, Ishiura S : Muscleblind protein, MBNL1/EXP, binds specificallyto CHHG repeats. Hum Mol Genet 2004; 3: 495–507.
Napierela M, Krzyzosiak WJ : CUG repeats present in myotonin kinase RNA form metastable ‘slippery’ hairpins. J Biol Chem 1997; 272: 31079–31085.
Tian B, White RJ, Xia T et al: Expanded CUG repeat RNAs form hairpins that activate the double-stranded RNA-dependent protein kinase PKR. RNA 2000; 6: 79–87.
Hou MH, Lin SB, Yuann JM, Lin WC, Wang AH, Kan LL : Effects of polyamines on the thermal stability and formation kinetics of DNA duplexes with abnormal structure. Nucleic Acids Res 2001; 29: 5121–5128.
Venkiteswaran S, Vijayanathan V, Shirahata A, Thomas T, Thomas TJ : Antisense recognition of the HER-2 mRNA: effects of phosphorothioate substitution and polyamines on DNA.RNA, RNA.RNA, and DNA.DNA duplex stability. Biochemistry 2005; 44: 303–312.
Hieronymus H, Silver PA : A systems view of mRNP biology. Genes Dev 2004; 18: 2845–2860.
Moore MJ : From birth to death: the complex lives of eukaryotic mRNAs. Science 2005; 309: 1514–1518.
Portela A, Digard P : The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J Gen Virol 2002; 83: 723–734.
Sang BC, Gray DM : Fd gene 5 protein binds to double-stranded polydeoxyribonucleotides poly(dA.dT) and poly[d(A-T).d(A-T)]. Biochemistry 1987; 26: 7210–7214.
Pant K, Karpel RL, Rouzina I, Williams MC : Salt dependent binding of T4 gene 32 protein to single and double-stranded DNA: single molecule force spectroscopy measurements. J Mol Biol 2005; 349: 317–330.
Saminathan M, Antony T, Shirahata A, Sigal LH, Thomas T, Thomas TJ : Ionic and structural specificity effects of natural and synthetic polyamines on the aggregation and resolubilization of single-, double-, and triple-stranded DNA. Biochemistry 1999; 38: 3821–3830.
Finkelman FD, Madden KB, Morris SC et al: Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine–anti-cytokine antibody complexes. J Immunol 1993; 151: 1235–1244.
Lin X, Miller JW, Mankodi A et al: Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum Mol Genet 2006; 15: 2087–2097.
Timchenko LT : Reversal of fortune. Nat Genet 2006; 38: 976–977.
Kuyumcu-Martinez NM, Wang G-S, Cooper TA : Increased steady-state levels of CUGBP1 in myotonic dystrophy are due to PKC-mediated hyperphosphorylation. Mol Cell 2007; 28: 68–78.
Mankodi A, Logigian E, Callahan L et al: Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 2000; 289: 1769–1773.
Seznec H, Agbulut O, Sergeant N et al: Mice transgenic for the human myotonic dystrophy region with expanded CTG repeats display muscular and brain abnormalities. Hum Mol Genet 2001; 10: 2717–2726.
Kanadia RN, Shin J, Yuan Y et al: Reversal of RNA missplicing and myotonia after muscleblind overexpression in a mouse poly(CUG) model for myotonic dystrophy. Proc Natl Acad Sci USA 2006; 103: 11748–11753.
Acknowledgements
I acknowledge personal communications, thoughtful comments and generous sharing of unpublished data by Drs Thomas Cooper, Yoshihiro Kino, Wlodzimierz Krzyzosiak, Mani Mahadevan, Maurice Swanson and Lubov Timchenko. However, to absolve all of any responsibility for the views expressed in this article, I state that they are solely my own. This paper was presented at the New England Chapter Muscular Dystrophy Association Annual Conference, Rhode Island Hospital, Providence on 30 November 2007. Preparation of this article was supported in part by the Association Française contre les Myopathies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary information
Rights and permissions
About this article
Cite this article
Junghans, R. Dystrophia myotonia: why focus on foci?. Eur J Hum Genet 17, 543–553 (2009). https://doi.org/10.1038/ejhg.2008.227
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2008.227
Keywords
This article is cited by
-
Progress in therapeutic antisense applications for neuromuscular disorders
European Journal of Human Genetics (2010)
-
Clinical Ramifications of the MHC Family Fc Receptor FcRn
Journal of Clinical Immunology (2010)