Abstract
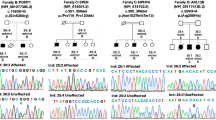
To date, six genes are known to cause nemaline (rod) myopathy (NM), a rare congenital neuromuscular disorder. In an attempt to find a seventh gene, we performed linkage and subsequent sequence analyses in 12 Turkish families with recessive NM. We found homozygosity in two of the families at 1q12-21.2, a region encompassing the γ-tropomyosin gene (TPM3) encoding slow skeletal muscle α-tropomyosin, a known NM gene. Sequencing revealed homozygous deletion of the first nucleotide of the last exon, c.913delA of TPM3 in both families. The mutation removes the last nucleotide before the stop codon, causing a frameshift and readthrough across the termination signal. The encoded αTmslow protein is predicted to be 73 amino acids longer than normal, and the extension to the protein is hypothesised to be unable to form a coiled coil. The resulting tropomyosin protein may therefore be non-functional. The affected children in both families were homozygous for the mutation, while the healthy parents were mutation carriers. Both of the patients in Family 1 had the severe form of NM, and also an unusual chest deformity. The affected children in Family 2 had the intermediate form of NM. Muscle biopsies showed type 1 (slow) fibres to be markedly smaller than type 2 (fast) fibres. Previously, there had been five reports, only, of NM caused by mutations in TPM3. The mutation reported here is the first deletion to be identified in TPM3, and it is likely to be a founder mutation in the Turkish population.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Shy GM, Engel WK, Somers JE, Wanko T : Nemaline myopathy: a new congenital myopathy. Brain 1963; 86: 793–810.
Wallgren-Pettersson C, Pelin K, Hilpela P et al: Clinical and genetic heterogeneity in autosomal recessive nemaline myopathy. Neuromuscul Disord 1999; 9: 564–572.
Wallgren-Pettersson C, Laing NG : Report of the 70th ENMC International Workshop: nemaline myopathy, 11–13 June 1999, Naarden, The Netherlands. Neuromuscul Disord 2000; 10: 299–306.
Pelin K, Hilpela P, Donner K et al: Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci USA 1999; 96: 2305–2310.
Nowak KJ, Wattanasirichaigoon D, Goebel HH et al: Mutations in the skeletal muscle alpha-actin gene in patients with actin myopathy and nemaline myopathy. Nat Genet 1999; 23: 208–212.
Laing NG, Wilton SD, Akkari PA et al: A mutation in the alpha tropomyosin gene TPM3 associated with autosomal dominant nemaline myopathy. Nat Genet 1995; 9: 75–79.
Donner K, Ollikainen M, Ridanpaa M et al: Mutations in the beta-tropomyosin (TPM2) gene–a rare cause of nemaline myopathy. Neuromuscul Disord 2002; 12: 151–158.
Johnston JJ, Kelley RI, Crawford TO et al: A novel nemaline myopathy in the Amish caused by a mutation in troponin T1. Am J Hum Genet 2000; 67: 814–821.
Agrawal PB, Greenleaf RS, Tomczak KK et al: Nemaline myopathy with minicores caused by mutation of the CFL2 gene encoding the skeletal muscle actin-binding protein, cofilin-2. Am J Hum Genet 2007; 80: 162–167.
Monnier N, Romero NB, Lerale J et al: An autosomal dominant congenital myopathy with cores and rods is associated with a neomutation in the RYR1 gene encoding the skeletal muscle ryanodine receptor. Hum Mol Genet 2000; 9: 2599–2608.
Scacheri PC, Hoffman EP, Fratkin JD et al: A novel ryanodine receptor gene mutation causing both cores and rods in congenital myopathy. Neurology 2000; 55: 1689–1696.
Gommans IM, Davis M, Saar K et al: A locus on chromosome 15q for a dominantly inherited nemaline myopathy with core-like lesions. Brain 2003; 126: 1545–1551.
Wattanasirichaigoon D, Swoboda KJ, Takada F et al: Mutations of the slow muscle alpha-tropomyosin gene, TPM3, are a rare cause of nemaline myopathy. Neurology 2002; 59: 613–617.
Parry DA : Coiled-coils in alpha-helix-containing proteins: analysis of the residue types within the heptad repeat and the use of these data in the prediction of coiled-coils in other proteins. Biosci Rep 1982; 2: 1017–1024.
Lupas A, Van Dyke M, Stock J : Predicting coiled coils from protein sequences. Science 1991; 252: 1162–1164.
Lupas A : Coiled coils: new structures and new functions. Trends Biochem Sci 1996; 21: 375–382.
Tan P, Briner J, Boltshauser E et al: Homozygosity for a nonsense mutation in the alpha-tropomyosin slow gene TPM3 in a patient with severe infantile nemaline myopathy. Neuromuscul Disord 1999; 9: 573–579.
Penisson-Besnier I, Monnier N, Toutain A, Dubas F, Laing N : A second pedigree with autosomal dominant nemaline myopathy caused by TPM3 mutation: a clinical and pathological study. Neuromuscul Disord 2007; 17: 330–337.
Dufour C, Weinberger RP, Schevzov G, Jeffrey PL, Gunning P : Splicing of two internal and four carboxyl-terminal alternative exons in nonmuscle tropomyosin 5 pre-mRNA is independently regulated during development. J Biol Chem 1998; 273: 18547–18555.
Cooper JA : Actin dynamics: tropomyosin provides stability. Curr Biol 2002; 12: R523–R525.
Gordon AM, Homsher E, Regnier M : Regulation of contraction in striated muscle. Physiol Rev 2000; 80: 853–924.
Pittenger MF, Kazzaz JA, Helfman DM : Functional properties of non-muscle tropomyosin isoforms. Curr Opin Cell Biol 1994; 6: 96–104.
Dufour C, Weinberger RP, Gunning P : Tropomyosin isoform diversity and neuronal morphogenesis. Immunol Cell Biol 1998; 5: 424–429.
Cooley BC, Bergtrom G : Multiple combinations of alternatively spliced exons in rat tropomyosin-alpha gene mRNA: evidence for 20 new isoforms in adult tissues and cultured cells. Arch Biochem Biophys 2001; 390: 71–77.
Gunning PW, Schevzov G, Kee AJ, Hardeman EC : Tropomyosin isoforms: divining rods for actin cytoskeleton function. Trends Cell Biol 2005; 15: 333–341.
Durling HJ, Reilich P, Muller-Hocker J et al: De novo missense mutation in a constitutively expressed exon of the slow alpha-tropomyosin gene TPM3 associated with an atypical, sporadic case of nemaline myopathy. Neuromuscul Disord 2002; 12: 947–951.
Clarke N, Kolski H, Dye D et al: Mutations in TPM3 are a common cause of congenital fiber type disproportion. Ann Neurol (in press).
Sung SS, Brassington AM, Grannatt K et al: Mutations in genes encoding fast-twitch contractile proteins cause distal arthrogryposis syndromes. Am J Hum Genet 2003; 72: 681–690.
Lehtokari VL, Ceuterick-de Groote C, de Jonghe P et al: Cap disease caused by heterozygous deletion of the beta-tropomyosin gene TPM2. Neuromuscul Disord 2007; 17: 433–442.
Thierfelder L, Watkins H, MacRae C et al: Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell 1994; 77: 701–712.
Watkins H, Anan R, Coviello DA, Spirito P, Seidman JG, Seidman CE : A de novo mutation in alpha-tropomyosin that causes hypertrophic cardiomyopathy. Circulation 1995; 91: 2302–2305.
Karibe A, Tobacman LS, Strand J et al: Hypertrophic cardiomyopathy caused by a novel alpha-tropomyosin mutation (V95A) is associated with mild cardiac phenotype, abnormal calcium binding to troponin, abnormal myosin cycling, and poor prognosis. Circulation 2001; 103: 65–71.
Greenfield N, Huang Y, Swapna G et al: Solution NMR structure of the junction between tropomyosin molecules: implications for actin binding and regulation. J Mol Biol 2006; 364: 80–96.
Wang J, Sanger J, Kang S et al: Ectopic expression and dynamics of TPM1alpha and TPM1kappa in myofibrils of avian myotubes. Cell Motil Cytoskeleton 2007; 64: 767–776.
Jeannet PY, Mittaz L, Dunand M, Lobrinus JA, Bonafe L, Kuntzer T : Autosomal dominant nemaline myopathy: a new phenotype unlinked to previously known genetic loci. Neuromuscul Disord 2007; 17: 6–12.
Acknowledgements
We thank Drs B Hamel, H Kingston, M Lammens, C Müller-Reible, H Marschner-Schäfer, F Muntoni, Y Nevo, E Oberstzyn, S Reus and D Wenger for DNA samples from Turkish patients with nemaline myopathy in whom the mutation was not found. Marilotta Turunen and Dan Brudzewsky are thanked for technical assistance. Jaakko Sarparanta is acknowledged for kind help with figures.
Funding: VLL was supported by grants to CWP from the University of Helsinki, the Association Francaise contre les Myopathies, the Sigrid Jusélius Foundation, the Academy of Finland, the Finska Läkaresällskapet, and the Medicinska understödsföreningen Liv och Hälsa; KP was supported by grants from the University of Helsinki, the Association Francaise contre les Myopathies and the Oskar Öflund Foundation; AHB was supported by NIH AR44345, the Muscular Dystrophy Association of the USA, and the Joshua Frase Foundation. NGL was supported by Australian National Health and Medical Research Council (NH and MRC) Fellowship 403904.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lehtokari, VL., Pelin, K., Donner, K. et al. Identification of a founder mutation in TPM3 in nemaline myopathy patients of Turkish origin. Eur J Hum Genet 16, 1055–1061 (2008). https://doi.org/10.1038/ejhg.2008.60
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2008.60
Keywords
This article is cited by
-
Tropomyosin 3 (TPM3) function in skeletal muscle and in myopathy
Skeletal Muscle (2023)
-
Transcript-Based Diagnosis and Expanded Phenotype of an Intronic Mutation in TPM3 Myopathy
Molecular Diagnosis & Therapy (2022)
-
Nemaline myopathies: a current view
Journal of Muscle Research and Cell Motility (2019)
-
Chronic Megacolon Presenting in Adolescents or Adults: Clinical Manifestations, Diagnosis, and Genetic Associations
Digestive Diseases and Sciences (2019)
-
Congenital myopathies: disorders of excitation–contraction coupling and muscle contraction
Nature Reviews Neurology (2018)