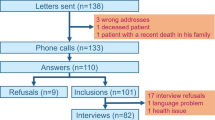
Abstract
Germline mutations in SDHD predispose to the development of head and neck paragangliomas, and phaeochromocytomas. The risk of developing a tumor depends on the sex of the parent who transmits the mutation: paragangliomas only arise upon paternal transmission. In this study, both the risk of paraganglioma and phaeochromocytoma formation, and the risk of developing associated symptoms were investigated in 243 family members with the SDHD.D92Y founder mutation. By using the Kaplan–Meier method, age-specific penetrance was calculated separately for paraganglioma formation as defined by magnetic resonance imaging (MRI) and for paraganglioma-related signs and symptoms. Evaluating clinical signs and symptoms alone, the penetrance reached a maximum of 57% by the age of 47 years. When MRI detection of occult paragangliomas was included, penetrance was estimated to be 54% by the age of 40 years, 68% by the age of 60 years and 87% by the age of 70 years. Multiple tumors were found in 65% and phaeochromocytomas were diagnosed in 8% of paraganglioma patients. Malignant paraganglioma was diagnosed in one patient (3%). Although the majority of carriers of a paternally inherited SDHD mutation will eventually develop head and neck paragangliomas, we find a lower penetrance than previous estimates from studies based on predominantly index cases. The family-based study described here emphasizes the importance of the identification and inclusion of clinically unaffected mutation carriers in all estimates of penetrance. This finding will allow a more accurate genetic counseling and warrants a ‘wait and scan’ policy for asymptomatic paragangliomas, combined with biochemical screening for catecholamine excess in SDHD-linked patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Parry DM, Li FP, Strong LC et al: Carotid body tumors in humans: genetics and epidemiology. J Natl Cancer Inst 1982; 68: 573–578.
Jansen JC, van den Berg R, Kuiper A, van der Mey AG, Zwinderman AH, Cornelisse CJ : Estimation of growth rate in patients with head and neck paragangliomas influences the treatment proposal. Cancer 2000; 88: 2811–2816.
McCaffrey TV, Meyer FB, Michels VV, Piepgras DG, Marion MS : Familial paragangliomas of the head and neck. Arch Otolaryngol Head Neck Surg 1994; 120: 1211–1216.
Struycken PM, Cremers CWRJ, Mariman ECM, Joosten FBM, Bleker RJTM : Glomus tumours and genomic imprinting: influence of inheritance along the paternal or maternal line. Clin Otolaryngol 1997; 22: 71–76.
Astuti D, Latif F, Dallol A et al: Mutations in the mitochondrial complex II subunit SDHB cause susceptibility to familial phaeochromocytoma and paraganglioma. J Med Genet 2001; 38: S22.
Niemann S, Muller U : Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet 2000; 26: 268–270.
Baysal BE, Ferrell RE, Willett-Brozick JE et al: Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000; 287: 848–851.
Mariman ECM, van Beersum SEC, Cremers CWRJ, van Baars FM, Ropers HH : Analysis of a second family with hereditary non-chromaffin paragangliomas locates the underlying gene at the proximal region of chromosome-11Q. Hum Genet 1993; 91: 357–361.
Peczkowska M, Cascon A, Prejbisz A et al: Extra-adrenal and adrenal pheochromocytomas associated with a germline SDHC mutation. Nat Clin Pract Endocrinol Metab 2008; 4: 111–115.
van Houtum WH, Corssmit EP, Douwes Dekker PB et al: Increased prevalence of catecholamine excess and phaeochromocytomas in a well-defined Dutch population with SDHD-linked head and neck paragangliomas. Eur J Endocrinol 2005; 152: 87–94.
van Schothorst EM, Jansen JC, Grooters E et al: Founder effect at PGL1 in hereditary head and neck paraganglioma families from the Netherlands. Am J Hum Genet 1998; 63: 468–473.
Astuti D, Latif F, Dallol A et al: Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet 2001; 69: 49–54.
van der Mey AGL, Maaswinkelmooy PD, Cornelisse CJ, Schmidt PH, van de Kamp JJP : Genomic imprinting in hereditary glomus tumors – evidence for new genetic theory. Lancet 1989; 2: 1291–1294.
Mariman ECM, van Beersum SEC, Cremers CWRJ, Struycken PM, Ropers HH : Fine mapping of a putatively imprinted gene for familial nonchromaffin paragangliomas to chromosome 11Q13.1 – evidence for genetic-heterogeneity. Hum Genet 1995; 95: 56–62.
Neumann HP, Pawlu C, Peczkowska M et al: Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 2004; 292: 943–951.
Benn DE, Gimenez-Roqueplo AP, Reilly JR et al: Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab 2006; 91: 827–836.
Gayther SA, Mangion J, Russell P et al: Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet 1997; 15: 103–105.
van Gils AP, van der Mey AG, Hoogma RP et al: MRI screening of kindred at risk of developing paragangliomas: support for genomic imprinting in hereditary glomus tumours. Br J Cancer 1992; 65: 903–907.
Oosterwijk JC, Jansen JC, van Schothorst EM et al: First experiences with genetic counselling based on predictive DNA diagnosis in hereditary glomus tumours (paragangliomas). J Med Genet 1996; 33: 379–383.
van Schothorst EM, Jansen JC, Bardoel AF et al: Confinement of PGL, an imprinted gene causing hereditary paragangliomas, to a 2-cM interval on 11q22–q23 and exclusion of DRD2 and NCAM as candidate genes. Eur J Hum Genet 1996; 4: 267–273.
Taschner PEM, Jansen JC, Baysal BE et al: Nearly all hereditary paragangliomas in the Netherlands are caused by two founder mutations in the SDHD gene. Genes Chromosomes Cancer 2001; 31: 274–281.
Hensen EF, Jordanova ES, van Minderhout IJHM et al: Somatic loss of maternal chromosome 11 causes parent-of-origin-dependent inheritance in SDHD-linked paraganglioma and phaeochromocytoma families. Oncogene 2004; 23: 4076–4083.
van Baars FM, Cremers CWRJ, van den Broek P, Veldman JE : Familiar non-chromaffinic paragangliomas (glomus tumors) – clinical and genetic-aspects (abridged). Acta Otolaryngol 1981; 91: 589–593.
Pigny P, Vincent A, Cardot BC et al: Paraganglioma after maternal transmission of a succinate dehydrogenase gene mutation. J Clin Endocrinol Metab 2008; 93: 1609–1615.
Gong G, Whittemore AS : Optimal designs for estimating penetrance of rare mutations of a disease-susceptibility gene. Genet Epidemiol 2003; 24: 173–180.
Baysal BE : Genomic imprinting and environment in hereditary paraganglioma. Am J Med Genet C 2004; 129C: 85–90.
Choi YH, Kopciuk KA, Briollais L : Estimating disease risk associated with mutated genes in family-based designs. Hum Hered 2008; 66: 238–251.
Begg CB : On the use of familial aggregation in population-based case probands for calculating penetrance. J Natl Cancer Inst 2002; 94: 1221–1226.
Astrom K, Cohen JE, Willett-Brozick JE, Aston CE, Baysal BE : Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Hum Genet 2003; 113: 228–237.
Arias-Stella J, Valcarcel J : Chief cell hyperplasia in the human carotid body at high altitudes. Hum Pathol 1976; 7: 361–373.
Havekes B, Corssmit EP, Jansen JC, van der Mey AG, Vriends AH, Romijn JA : Malignant paragangliomas associated with mutations in the succinate dehydrogenase D gene. J Clin Endocrinol Metab 2007; 92: 1245–1248.
Dekker PBD, Hogendoorn PCW, Kuipers-Dijkshoorn N et al: SDHD mutations in head and neck paragangliomas result in destabilization of complex II in the mitochondrial respiratory chain with loss of enzymatic activity and abnormal mitochondrial morphology. J Pathol 2003; 201: 480–486.
Zeegers MPA, van Poppel F, Vlietinck R, Spruijt L, Ostrer H : Founder mutation among the Dutch. Eur J Hum Genet 2004; 12: 591–600.
Acknowledgements
The authors would like to thank Marciano B Ferrier and Ekaterina S Jordanova for their help with the statistical analysis and the figures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hensen, E., Jansen, J., Siemers, M. et al. The Dutch founder mutation SDHD.D92Y shows a reduced penetrance for the development of paragangliomas in a large multigenerational family. Eur J Hum Genet 18, 62–66 (2010). https://doi.org/10.1038/ejhg.2009.112
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2009.112
Keywords
This article is cited by
-
Clinical progression and metachronous paragangliomas in a large cohort of SDHD germline variant carriers
European Journal of Human Genetics (2018)
-
High prevalence of occult paragangliomas in asymptomatic carriers of SDHD and SDHB gene mutations
European Journal of Human Genetics (2013)
-
Where genotype is not predictive of phenotype: towards an understanding of the molecular basis of reduced penetrance in human inherited disease
Human Genetics (2013)
-
Recent advances in the genetics of SDH-related paraganglioma and pheochromocytoma
Familial Cancer (2011)