Abstract
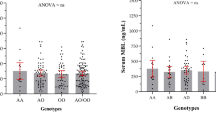
Variants in mannose-binding lectin (MBL2; protein MBL) have shown association with different aspects (eg, lung function, infection, survival) of cystic fibrosis (CF) in some studies but not others. Inconsistent results may be due to confounding among disease variables that were not fully accounted for in each study. To account for these relationships, we derived a modeling framework incorporating CFTR genotype, age, Pseudomonas aeruginosa (Pa) infection, and lung function from 788 patients in the US CF Twin and Sibling Study. This framework was then used to identify confounding variables when testing the effect of MBL2 variation on specific CF traits. MBL2 genotypes corresponding to low levels of MBL associated with Pa infection 1.94 years earlier than did MBL2 genotypes corresponding to high levels of MBL (P=0.0034). In addition, Pa-infected patients with MBL2 genotypes corresponding to low levels of MBL underwent conversion to mucoid Pa 2.72 years earlier than did patients with genotypes corresponding to high levels of MBL (P=0.0003). MBL2 was not associated with the time to transition from infection to conversion or with lung function. Thus, use of a modeling framework that identified confounding among disease variables revealed that variation in MBL2 associates with age at infection with Pa and age at conversion to mucoid Pa in CF.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Vanscoy LL, Blackman SM, Collaco JM et al: Heritability of lung disease severity in cystic fibrosis. Am J Respir Crit Care Med 2007; 10: 1036–1043.
Garred P, Pressler T, Madsen HO et al: Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest 1999; 104: 431–437.
Ohlenschlaeger T, Garred P, Madsen HO, Jacobsen S : Mannose-binding lectin variant alleles and the risk of arterial thrombosis in systemic lupus erythematosus. N Engl J Med 2004; 3: 260–267.
Horiuchi T, Gondo H, Miyagawa H et al: Association of MBL gene polymorphisms with major bacterial infection in patients treated with high-dose chemotherapy and autologous PBSCT. Genes Immun 2005; 2: 162–166.
Li Z, Kosorok MR, Farrell PM et al: Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 2005; 5: 581–588.
Buranawuti K, Boyle MP, Cheng S et al: Variants in mannose-binding lectin and tumor necrosis factor {alpha} affect survival in cystic fibrosis. J Med Genet 2006; 3: 209–214.
Carlsson M, Sjoholm AG, Eriksson L et al: Deficiency of the mannan-binding lectin pathway of complement and poor outcome in cystic fibrosis: bacterial colonization may be decisive for a relationship. Clin Exp Immunol 2005; 2: 306–313.
Dorfman R, Sandford A, Taylor C et al: Complex two-gene modulation of lung disease severity in children with cystic fibrosis. J Clin Invest 2008; 3: 1040–1049.
Trevisiol C, Boniotto M, Giglio L, Poli F, Morgutti M, Crovella S : MBL2 polymorphisms screening in a regional Italian CF center. J Cyst Fibros 2005; 3: 189–191.
Gabolde M, Guilloud-Bataille M, Feingold J, Besmond C : Association of variant alleles of mannose binding lectin with severity of pulmonary disease in cystic fibrosis: cohort study. BMJ 1999; 7218: 1166–1167.
Davies JC, Turner MW, Klein N : Impaired pulmonary status in cystic fibrosis adults with two mutated MBL-2 alleles. Eur Respir J 2004; 5: 798–804.
Muhlebach MS, MacDonald SL, Button B et al: Association between mannan-binding lectin and impaired lung function in cystic fibrosis may be age-dependent. Clin Exp Immunol 2006; 2: 302–307.
Yarden J, Radojkovic D, De Boeck K et al: Polymorphisms in the mannose binding lectin gene affect the cystic fibrosis pulmonary phenotype. J Med Genet 2004; 8: 629–633.
Olesen HV, Jensenius JC, Steffensen R, Thiel S, Schiotz PO : The mannan-binding lectin pathway and lung disease in cystic fibrosis--dysfunction of mannan-binding lectin-associated serine protease 2 (MASP-2) may be a major modifier. Clin Immunol 2006; 3: 324–331.
Drumm ML, Konstan MW, Schluchter MD et al: Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med 2005; 14: 1443–1453.
Cutting GR : Modifier genetics: cystic fibrosis. Annu Rev Genomics Hum Genet 2005; 6: 237–260.
Colhoun HM, McKeigue PM, Davey SG : Problems of reporting genetic associations with complex outcomes. Lancet 2003; 9360: 865–872.
Greenland S, Morgenstern H : Confounding in health research. Annu Rev Public Health 2001; 22: 189–212.
Vanscoy LL, Lai T, Bowers A, Cutting GR : Modifier genes may contribute substantially to variation in cystic fibrosis (CF) lung disease estimated by CF-specific % for FEV1. Proc Am Thorac Soc 2006; 3: A408.
Cystic Fibrosis Foundation: Cystic Fibrosis Foundation Patient Registry Annual Data Report 2006, Bethesda, MD: Cystic Fibrosis Foundation, 2006.
Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B : Changes in the normal maximal expiratory flow–volume curve with growth and aging. Am Rev Respir Dis 1983; 6: 725–734.
Kulich M, Rosenfeld M, Campbell J et al: Disease-specific reference equations for lung function in patients with cystic fibrosis. Am J Respir Crit Care Med 2005; 7: 885–891.
Sumiya M, Super M, Tabona P et al: Molecular basis of opsonic defect in immunodeficient children. Lancet 1991; 8757: 1569–1570.
Lipscombe RJ, Sumiya M, Hill AV et al: High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet 1992; 9: 709–715.
Madsen HO, Garred P, Kurtzhals JA et al: A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics 1994; 1: 37–44.
Madsen HO, Garred P, Thiel S et al: Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol 1995; 6: 3013–3020.
Baxter N, Sumiya M, Cheng S et al: Recurrent miscarriage and variant alleles of mannose binding lectin, tumour necrosis factor and lymphotoxin alpha genes. Clin Exp Immunol 2001; 3: 529–534.
Wang X, Myers A, Saiki RK, Cutting GR : Development and evaluation of a PCR-based, line probe assay for the detection of 58 alleles in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Clin Chem 2002; 7: 1121–1123.
Bremer LA, Blackman SM, Vanscoy LL et al: Interaction between a novel TGFB1 haplotype and CFTR genotype is associated with improved lung function in cystic fibrosis. Hum Mol Genet 2008.
Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA : Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 2002; 2: 176–184.
O’Brien RG : A tour of UnifyPow: a SAS module/macro for sample-size analysis. Proceedings of the 23rd Annual SAS Users Group International Conference, Cary, NC 1998; 1346–1355.
Collaco JM, Vanscoy L, Bremer L et al: Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA 2008; 4: 417–424.
Blackman SM, Deering-Brose R, McWilliams R et al: Relative contribution of genetic and nongenetic modifiers to intestinal obstruction in cystic fibrosis. Gastroenterology 2006; 4: 1030–1039.
Renders NH, Sijmons MA, van Belkum A, Overbeek SE, Mouton JW, Verbrugh HA : Exchange of Pseudomonas aeruginosa strains among cystic fibrosis siblings. Res Microbiol 1997; 5: 447–454.
Thomassen MJ, Demko CA, Doershuk CF, Root JM : Pseudomonas aeruginosa isolates: comparisons of isolates from campers and from sibling pairs with cystic fibrosis. Pediatr Pulmonol 1985; 1: 40–45.
Nixon GM, Armstrong DS, Carzino R et al: Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr 2001; 5: 699–704.
Kosorok MR, Zeng L, West SE et al: Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol 2001; 4: 277–287.
Ballmann M, Rabsch P, von der HH : Long-term follow up of changes in FEV1 and treatment intensity during Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Thorax 1998; 9: 732–737.
Acknowledgements
We thank the North American Cystic Fibrosis Foundation for use of the Cystic Fibrosis Foundation Data Registry; Ase Sewall, Monica Brooks (both from Cystic Fibrosis Foundation), and the staff at the Data Registry; Nulang Wang for CFTR genotyping; Erica Senat for her assistance with MBL2 genotyping; Michael Kulich (Charles University, Prague, Czech Republic), for providing conversion programs for cystic fibrosis-specific percentiles; Rita McWilliams, Julie Hoover-Fong, and Ada Hamosh, all from Johns Hopkins University, for designing questionnaires; and most importantly the patients with cystic fibrosis and their families, research coordinators, nurses, and physicians participating in the Cystic Fibrosis Twin and Sibling Study. This study was supported by the National Heart, Lung, and Blood Institute Grant HL68927. Genotyping reagents were provided by Roche Molecular Systems Inc (Pleasanton, CA, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
GR Cutting is a consultant for Roche Molecular Systems, and received less than US$1000 per year as consulting fees. M Grow and S Cheng are employed by Roche Molecular Systems.
Rights and permissions
About this article
Cite this article
McDougal, K., Green, D., Vanscoy, L. et al. Use of a modeling framework to evaluate the effect of a modifier gene (MBL2) on variation in cystic fibrosis. Eur J Hum Genet 18, 680–684 (2010). https://doi.org/10.1038/ejhg.2009.226
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2009.226