Abstract
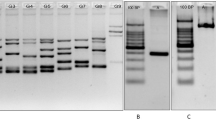
Preimplantation Genetic Diagnosis (PGD) is a method of testing in vitro embryos as an alternative to prenatal diagnosis with possible termination of pregnancy in case of an affected child. Recently, PGD for hereditary breast and ovarian cancer caused by BRCA1 and BRCA2 mutations has found its way in specialized labs. We describe the route to universal single-cell PGD tests for carriers of BRCA1/2 mutations. Originally, mutation-specific protocols with one or two markers were set up and changed when new couples were not informative. This route of changing protocols was finalized after 2 years with universal tests for both BRCA1 and BRCA2 mutation carriers based on haplotyping of, respectively, 6 (BRCA1) and 8 (BRCA2) microsatellite markers in a multiplex PCR. Using all protocols, 30 couples had a total of 47 PGD cycles performed. Eight cycles were cancelled upon IVF treatment due to hypostimulation. Of the remaining 39 cycles, a total of 261 embryos were biopsied and a genetic diagnosis was obtained in 244 (93%). In 34 of the 39 cycles (84.6%), an embryo transfer was possible and resulted in 8 pregnancies leading to a fetal heart beat per oocyte retrieval of 20.5% and a fetal heart beat per embryonic transfer of 23.5%. The preparation time and costs for set-up and validation of tests are minimized. The informativity of microsatellite markers used in the universal PGD-PCR tests is based on CEPH and deCODE pedigrees, making the tests applicable in 90% of couples coming from these populations.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Devilee P, Tollenaar RA, Cornelisse CJ : [From gene to disease; from BRCA1 or BRCA2 to breast cancer]. Ned Tijdschr Geneeskd 2000; 144: 2549–2551.
Tutt A, Ashworth A : The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med 2002; 8: 571–576.
Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE : Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 1994; 343: 692–695.
Wooster R, Bignell G, Lancaster J et al: Identification of the breast cancer susceptibility gene BRCA2. Nature 1995; 378: 789–792.
Abeliovich D, Kaduri L, Lerer I et al: The founder mutations 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 appear in 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women. Am J Human Genet 1997; 60: 505–514.
Borg A, Dorum A, Heimdal K, Maehle L, Hovig E, Moller P : BRCA1 1675delA and 1135insA account for one third of Norwegian familial breast-ovarian cancer and are associated with later disease onset than less frequent mutations. Dis Markers 1999; 15: 79–84.
Gorski B, Jakubowska A, Huzarski T et al: A high proportion of founder BRCA1 mutations in Polish breast cancer families. IntJ Cancer 2004; 110: 683–686.
Khoo US, Chan KY, Cheung AN et al: Recurrent BRCA1 and BRCA2 germline mutations in ovarian cancer: a founder mutation of BRCA1 identified in the Chinese population. HumanMmutat 2002; 19: 307–308.
Verhoog LC, van den Ouweland AM, Berns E et al: Large regional differences in the frequency of distinct BRCA1/BRCA2 mutations in 517 Dutch breast and/or ovarian cancer families. Eur J Cancer 2001; 37: 2082–2090.
de Die-Smulders CE, Land JA, Dreesen JC, Coonen E, Evers JL, Geraedts JP : [Results from 10 years of preimplantation-genetic diagnostics in The Netherlands]. Ned Tijdschr Geneeskd 2004; 148: 2491–2496.
Menon U, Harper J, Sharma A et al: Views of BRCA gene mutation carriers on preimplantation genetic diagnosis as a reproductive option for hereditary breast and ovarian cancer. Hum Reprod 2007; 22: 1573–1577.
Sagi M, Weinberg N, Eilat A et al: Preimplantation genetic diagnosis for BRCA1/2—a novel clinical experience. Prenat Diagn 2009; 29: 508–513.
Spits C, De Rycke M, Van Ranst N et al: Preimplantation genetic diagnosis for cancer predisposition syndromes. Prenat Diagn 2007; 27: 447–456.
Kotsopoulos J, Librach CL, Lubinski J et al: Infertility, treatment of infertility, and the risk of breast cancer among women with BRCA1 and BRCA2 mutations: a case-control study. Cancer Causes Control 2008; 19: 1111–1119.
Moutou C, Gardes N, Nicod JC, Viville S : Strategies and outcomes of PGD of familial adenomatous polyposis. Mol Hum Reprod 2007; 13: 95–101.
Harton GL, De Rycke M, Fiorentino F et al: ESHRE PGD consortium best practice guidelines for amplification-based PGD. Hum Reprod 2011; 26: 33–40.
Dumoulin JC, Land JA, Van Montfoort AP et al: Effect of in vitro culture of human embryos on birthweight of newborns. Hum Reprod 2010; 25: 605–612.
Van Steirteghem AC, Nagy Z, Joris H et al: High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod 1993; 8: 1061–1066.
Dumoulin JC, Coonen E, Bras M et al: Comparison of in-vitro development of embryos originating from either conventional in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod 2000; 15: 402–409.
Thornhill AR, deDie-Smulders CE, Geraedts JP et al: ESHRE PGD Consortium 'Best practice guidelines for clinical preimplantation genetic diagnosis (PGD) and preimplantation genetic screening (PGS)’. Hum Reprod 2005; 20: 35–48.
Handyside AH : Preimplantation genetic diagnosis after 20 years. Reprod Biomed Online 2010; 21: 280–282.
Robertson JA : Extending preimplantation genetic diagnosis: the ethical debate. Ethical issues in new uses of preimplantation genetic diagnosis. Hum Reprod 2003; 18: 465–471.
Jasper MJ, Liebelt J, Hussey ND : Preimplantation genetic diagnosis for BRCA1 exon 13 duplication mutation using linked polymorphic markers resulting in a live birth. Prenat Diagn 2008; 28: 292–298.
Ramon YCT, Polo A, Martinez O et al: Preimplantation genetic diagnosis for inherited breast cancer: first clinical application and live birth in Spain. Familial Cancer 2012; 11: 175–179.
Renwick PJ, Trussler J, Ostad-Saffari E et al: Proof of principle and first cases using preimplantation genetic haplotyping—a paradigm shift for embryo diagnosis. Reprod Biomed Online 2006; 13: 110–119.
Acknowledgements
We thank other members of the PGD working group who contributed to the study: Marieke van Deursen, Yvonne Arens, Chantal Bastiaens, Celine Eggen, Nienke Muntjewerff, Guusje de Krom, Aisha de Graaff, Ewka Nelissen, Inge Schreurs, Marij Janssen, Mirjam Wolffs, Marion Meijs, Josien Derhaag, John Dumoulin, Sabine Spierts, Wim Loneus, Yens Jackers, Laurence Meers, Anneke de Vreeden-Elbertse, Yvonne Koot, Madelon Meijer-Hoogeveen, Irene Homminga, Elsbeth Dul and Jolande Land.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Drüsedau, M., Dreesen, J., Derks-Smeets, I. et al. PGD for hereditary breast and ovarian cancer: the route to universal tests for BRCA1 and BRCA2 mutation carriers. Eur J Hum Genet 21, 1361–1368 (2013). https://doi.org/10.1038/ejhg.2013.50
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2013.50
Keywords
This article is cited by
-
Fertility preservation in BRCA mutation carriers—efficacy and safety issues: a review
Reproductive Biology and Endocrinology (2020)
-
Ovarian stimulation for IVF and risk of primary breast cancer in BRCA1/2 mutation carriers
British Journal of Cancer (2018)
-
BRCA1 mutation carriers have a lower number of mature oocytes after ovarian stimulation for IVF/PGD
Journal of Assisted Reproduction and Genetics (2017)
-
Präimplantationsdiagnostik – methodische Aspekte
Medizinische Genetik (2016)
-
Advances in preimplantation genetic diagnosis/screening
Science China Life Sciences (2014)