Abstract
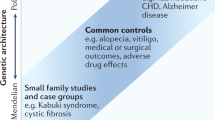
New genetic technologies are capable of returning far more information than the single answer to the single question posed when conducting a given genetic test. Genetics contexts consequently stand on the brink of an explosion of what have traditionally been called ‘incidental findings’. However, the continued use of this term is controversial. Various replacements for ‘incidental findings’ have been attempted, but none with widespread success. Agreement on terminology and definitions is vital so that the legal and ethical debate around incidental findings can proceed. We highlight the difficulties raised by the various terms currently used as alternatives, and end by defending our choice for the term ‘secondary variants’.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aron DC : Preface. Best Prac Res Clin Endocrinol Metabol 2012; 26: 1–2.
Mirilas P, Skandalakis JE : Benign anatomical mistakes: incidentaloma. Am Surg 2002; 68: 1026.
Stone JH : Incidentalomas — Clinical correlation and translational science required. N Engl J Med 2006; 354: 2748–2749.
Kohane IS, Hsing M, Kong SW : Taxonomizing, sizing, and overcoming the incidentalome. Genet Med 2012; 14: 399–404.
Wolf S : Introduction: the challenge of incidental findings. J Law Med Ethics 2008; 36: 216–218.
Wolf SM : The past, present, and future of the debate over return of research results and incidental findings. Genet Med 2012; 14: 355–357.
Lawrenz F, Sobotka S : Empirical analysis of current approaches to incidental findings. J Law Med Ethics 2008; 36: 249–255.
Zawati MH, Knoppers BM : International normative perspectives on the return of individual research results and incidental findings in genomic biobanks. Genet Med 2012; 14: 484–489.
Christenhusz GM, Devriendt K, Dierickx K : To tell or not to tell? A systematic review of ethical reflections on incidental findings arising in genetics contexts. Eur J Hum Genet 2013; 21: 248–255.
Knoppers BM, Dam A : Return of results: towards a lexicon? J Law Med Ethics 2011; 39: 577–582.
Knoppers B, Joly Y, Simard J, Durocher F : The emergence of an ethical duty to disclose genetic research results: international perspectives. Eur J Hum Genet 2006; 14: 1170–1178.
Ravitsky V, Wilfond B : Disclosing individual genetic results to research participants. AJOB 2006; 6: 8–17.
Wolf S, Lawrenz F, Nelson C et al: Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics 2008; 36: 219–248.
Parker L : The future of incidental findings: should they be viewed as benefits? J Law Med Ethics 2008; 36: 341–351.
Sharp H, Orr R : When ‘minimal risk’ research yields clinically-significant data, maybe the risks aren't so minimal. AJOB 2004; 4: W32–W36.
Lyon GJ : There is nothing ‘incidental’ about unrelated findings. Pers Med 2012; 9: 163–166.
Health Council of the Netherlands: The ‘Thousand-Dollar Genome’: an Ethical Exploration. The Hague: Centre for Ethics and Health, 2010.
Stoskopf MK : Observation and cogitation: how serendipity provides the building blocks of scientific discovery. ILAR J 2005; 46: 332–337.
Kolata G : Genes now tell doctors secrets they can’t utter. The New York Times 2012.
Bush LW, Rothenberg KH : Dialogues, dilemmas, and disclosures: genomic research and incidental findings. Genet Med 2012; 14: 293–295.
Bruno DL, Stark Z, Amor DJ et al: Extending the scope of diagnostic chromosome analysis: detection of single gene defects using high-resolution SNP microarrays. Hum Mutat 2011; 32: 1500–1506.
Mayer AN, Dimmock DP, Arca MJ et al: A timely arrival for genomic medicine. Genet Med 2011; 13: 195–196.
Bovenberg J, Meulenkamp T, Smets E, Gevers S : Biobank research: reporting results to individual participants. Eur J Health Law 2009; 16: 229–247.
Kirschen M, Jaworska A, Illes J : Subjects' expectations in neuroimaging research. J Magn Reson Imaging 2006; 23: 205–209.
Matsui K, Lie RK, Kita Y, Ueshima H : Ethics of future disclosure of individual risk information in a genetic cohort study: a survey of donor preferences. J Epidemiol 2008; 18: 217–224.
Shaw R, Senior C, Peel E et al: Ethical issues in neuroimaging health research: an IPA study with research participants. J Health Psychol 2008; 13: 1051–1059.
Cho MK : Understanding incidental findings in the context of genetics and genomics. J Law Med Ethics 2008; 36: 280–285.
Miller FG, Mello MM, Joffe S : Incidental findings in human subjects research: what do investigators owe research participants? J Law Med Ethics 2008; 36: 271–279.
Becker F, van El CG, Ibarreta D et al: Genetic testing and common disorders in a public health framework: how to assess relevance and possibilities. background document to the ESHG recommendations on genetic testing and common disorders. Eur J Hum Genet 2011; 19: S6–44.
Smerecnik CM, Mesters I, de Vries NK, de Vries H : Educating the general public about multifactorial genetic disease: applying a theory-based framework to understand current public knowledge. Genet Med 2008; 10: 251–258.
Lavieri R, Garner S : Ethical considerations in the communication of unexpected information with clinical implications. AJOB 2006; 6: 46–48.
Sonnenberg A : Personal view: 'don't ask, don't tell'--the undesirable consequences of incidental test results in gastroenterology. Aliment Pharmacol Ther 2004; 20: 381–387.
Christenhusz GM, Devriendt K, Vermeesch J, Dierickx K : Why genomics shouldn’t get too personal: In favor of filters: Re: invited comment by Holly K. Tabor et al. in American Journal of Medical Genetics Part A Volume 155. Am J Med Genet A 2012; 158A: 2641–2642.
Meacham M, Starks H, Burke W, Edwards K : Researcher perspectives on disclosure of incidental findings in genetic research. J Empir Res Hum Res Ethics 2010; 5: 31–41.
Johnston JJ, Rubinstein WS, Facio FM et al: Secondary variants in individuals undergoing exome sequencing: screening of 572 individuals identifies high-penetrance mutations in cancer-susceptibility genes. Am J Hum Genet 2012; 91: 97–108.
Acknowledgements
This work was supported by FWO Flanders, project number G029107 and G.0594.09. K Devriendt is senior clinical investigator of the FWO-Vlaanderen and of the K.O.O.R U.Z.Leuven. We thank the anonymous reviewers for their valuable comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Christenhusz, G., Devriendt, K. & Dierickx, K. Secondary variants – in defense of a more fitting term in the incidental findings debate. Eur J Hum Genet 21, 1331–1334 (2013). https://doi.org/10.1038/ejhg.2013.89
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2013.89
Keywords
This article is cited by
-
Points to consider for laboratories reporting results from diagnostic genomic sequencing
European Journal of Human Genetics (2018)
-
Incidental or secondary findings: an integrative and patient-inclusive approach to the current debate
European Journal of Human Genetics (2018)
-
Effect of Public Deliberation on Attitudes toward Return of Secondary Results in Genomic Sequencing
Journal of Genetic Counseling (2017)
-
Compare and contrast: a cross-national study across UK, USA and Greek experts regarding return of incidental findings from clinical sequencing
European Journal of Human Genetics (2016)
-
Konzepte zur Mitteilung genetischer Zusatzbefunde in der medizinischen Diagnostik und Forschung
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2015)