Abstract
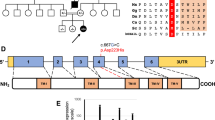
Isolated cytochrome c oxidase (COX) deficiency is a prevalent cause of mitochondrial disease and is mostly caused by nuclear-encoded mutations in assembly factors while rarely by mutations in structural subunits. We hereby report a case of isolated COX deficiency manifesting with encephalomyopathy, hydrocephalus and hypertropic cardiomyopathy due to a missense p.R20C mutation in the COX6B1 gene, which encodes an integral, nuclear-encoded COX subunit. This novel mutation was predicted to be severe in silico. In accord, enzymatic activity was undetectable in muscle and fibroblasts, was severely decreased in lymphocytes and the COX6B1 protein was barely detectable in patient’s muscle mitochondria. Complementation with the wild-type cDNA by a lentiviral construct restored COX activity, and mitochondrial function was improved by 5-aminoimidazole-4-carboxamide ribonucleotide, resveratrol and ascorbate in the patient’s fibroblasts. We suggest that genetic analysis of COX6B1should be included in the investigation of isolated COX deficiency, including patients with cardiac defects. Initial measurement of COX activity in lymphocytes may be useful as it might circumvent the need for invasive muscle biopsy. The evaluation of ascorbate supplementation to patients with mutated COX6B1 is warranted.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Di Donato S : Multisystem manifestations of mitochondrial disorders. J Neurol 2009; 256: 693–710.
DiMauro S, Tanji K, Schon EA : The many clinical faces of cytochrome c oxidase deficiency. Adv Exp Med Biol 2012; 748: 341–357.
Soto IC, Fontanesi F, Liu J, Barrientos A : Biogenesis and assembly of eukaryotic cytochrome c oxidase catalytic core. Biochim Biophys Acta 2012; 1817: 883–897.
Shteyer E, Saada A, Shaag A et al: Exocrine pancreatic insufficiency, dyserythropoeitic anemia, and calvarial hyperostosis are caused by a mutation in the COX4I2 gene. Am J Hum Genet 2009; 84: 412–417.
Indrieri A, van Rahden VA, Tiranti V et al: Mutations in COX7B cause microphthalmia with linear skin lesions, an unconventional mitochondrial disease. Am J Hum Genet 2012; 91: 942–949.
Massa V, Fernandez-Vizarra E, Alshahwan S et al: Severe infantile encephalomyopathy caused by a mutation in COX6B1, a nucleus-encoded subunit of cytochrome c oxidase. Am J Hum Genet 2008; 82: 1281–1289.
Saada A, Bar-Meir M, Belaiche C, Miller C, Elpeleg O : Evaluation of enzymatic assays and compounds affecting ATP production in mitochondrial respiratory chain complex I deficiency. Anal Biochem 2004; 335: 66–72.
Reisch AS, Elpeleg O : Biochemical assays for mitochondrial activity: assays of TCA cycle enzymes and PDHc. Methods Cell Biol 2007; 80: 199–222.
Darley-Usmar VM, Capaldi RA, Takamiya S et al: Reconstitution and molecular analysis of the respiratory chain; in Darley-Usmar VM, Rickwood D, Wilson MT (eds): Mitochondria a Practical Approach. Washington, DC, USA: IRL press, 1987, pp 113–152.
Muramoto K, Hirata K, Shinzawa-Itoh K et al: A histidine residue acting as a controlling site for dioxygen reduction and proton pumping by cytochrome c oxidase. Proc Natl Acad Sci USA 2007; 104: 7881–7886.
Kellogg EH, Leaver-Fay A, Baker D : Role of conformational sampling in computing mutation-induced changes in protein structure and stability. Proteins 2011; 79: 830–838.
Soiferman D, Ayalon O, Weissman S, Saada A : The effect of small molecules on nuclear-encoded translation diseases. Biochimie 2014; 100: 184–191.
Golubitzky A, Dan P, Weissman S, Link G, Wikstrom JD, Saada A : Screening for active small molecules in mitochondrial complex I deficient patient's fibroblasts, reveals AICAR as the most beneficial compound. PLoS One 2011; 6: e26883.
Gurgel-Giannetti J, Oliveira G, Brasileiro Filho G, Martins P, Vainzof M, Hirano M : Mitochondrial cardioencephalomyopathy due to a novel SCO2 mutation in a Brazilian patient: case report and literature review. JAMA Neurol 2013; 70: 258–261.
Zeharia A, Shaag A, Pappo O et al: Acute infantile liver failure due to mutations in the TRMU gene. Am J Hum Genet 2009; 85: 401–407.
Horvath R, Kemp JP, Tuppen HA et al: Molecular basis of infantile reversible cytochrome c oxidase deficiency myopathy. Brain 2009; 132: 3165–3174.
Martinelli D, Catteruccia M, Piemonte F et al: EPI-743 reverses the progression of the pediatric mitochondrial disease—genetically defined Leigh Syndrome. Mol Genet Metab 2012; 107: 383–388.
Salviati L, Hernandez-Rosa E, Walker WF et al: Copper supplementation restores cytochrome c oxidase activity in cultured cells from patients with SCO2 mutations. Biochem J 2002; 363: 321–327.
Casarin A, Giorgi G, Pertegato V et al: Copper and bezafibrate cooperate to rescue cytochrome c oxidase deficiency in cells of patients with SCO2 mutations. Orphanet J Rare Dis 2012; 19: 21.
Boczonadi V, Smith PM, Pyle A et al: Altered 2-thiouridylation impairs mitochondrial translation in reversible infantile respiratory chain deficiency. Hum Mol Genet 2013; 22: 4602–4615.
Sharma P, Mongan PD : Ascorbate reduces superoxide production and improves mitochondrial respiratory chain function in human fibroblasts with electron transport chain deficiencies. Mitochondrion 2001; 1: 191–198.
Ohlenbusch A, Edvardson S, Skorpen J et al: Leukoencephalopathy with accumulated succinate is indicative of SDHAF1 related complex II deficiency. Orphanet J Rare Dis 2012; 7: 69.
Acknowledgements
Corinne Belaiche is acknowledged for excellent technical assistance. This work was financed in part by E-Rare grant GenoMit (SE) and by the Manackerman Charitable Trust Fund UK (to DS and AS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Abdulhag, U., Soiferman, D., Schueler-Furman, O. et al. Mitochondrial complex IV deficiency, caused by mutated COX6B1, is associated with encephalomyopathy, hydrocephalus and cardiomyopathy. Eur J Hum Genet 23, 159–164 (2015). https://doi.org/10.1038/ejhg.2014.85
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2014.85
This article is cited by
-
The transcription regulator ATF4 is a mediator of skeletal muscle aging
GeroScience (2023)
-
Nicotinamide riboside kinase-2 regulates metabolic adaptation in the ischemic heart
Journal of Molecular Medicine (2023)
-
Characterization of terminal-ileal and colonic Crohn’s disease in treatment-naïve paediatric patients based on transcriptomic profile using logistic regression
Journal of Translational Medicine (2021)
-
Identification of key pathways and genes in polycystic ovary syndrome via integrated bioinformatics analysis and prediction of small therapeutic molecules
Reproductive Biology and Endocrinology (2021)
-
Mitochondrial complex IV deficiency caused by a novel frameshift variant in MT-CO2 associated with myopathy and perturbed acylcarnitine profile
European Journal of Human Genetics (2019)