Abstract
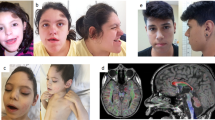
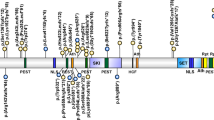
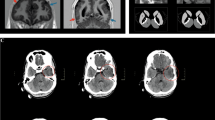
MED13L haploinsufficiency has recently been described as responsible for syndromic intellectual disability. We planned a search for causative gene variants in seven subjects with intellectual disability and overlapping dysmorphic facial features such as bulbous nasal tip, short mouth and straight eyebrows. We found two de novo frameshift variants in MED13L, consisting in single-nucleotide deletion (c.3765delC) and duplication (c.607dupT). A de novo nonsense variant (c.4420A>T) in MED13L was detected in a further subject in the course of routine whole-exome sequencing. By analyzing the clinical data of our patients along with those recently described in the literature, we confirm that there is a common, recognizable phenotype associated with MED13L haploinsufficiency, which includes intellectual disability and a distinctive facial appearance. Congenital heart diseases are found in some subjects with various degree of severity. Our observation of cleft palate, ataxia, epilepsy and childhood leukemia observed in single cases broadens the known clinical spectrum. Haploinsufficiency for MED13L should be considered in the differential diagnosis of the 1p36 microdeletion syndrome, due to overlapping dysmorphic facial features in some patients. The introduction of massive parallel-sequencing techniques into clinical practice is expected to allow for detection of other causative point variants in MED13L. Analysis of genomic data in connection with deep clinical evaluation of patients could elucidate genetic heterogeneity of the MED13L haploinsufficiency phenotype.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Curry CJ, Stevenson RE, Aughton D et al: Evaluation of mental retardation: recommendations of a Consensus Conference: American College of Medical Genetics. Am J Med Genet 1997; 72: 468–477.
Battaglia A, Carey JC : Diagnostic evaluation of developmental delay/mental retardation: an overview. Am J Med Genet C Semin Med Genet 2003; 117C: 3–14.
Leonard H, Wen X : The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment Retard Dev Disabil Res Rev 2002; 8: 117–134.
Zahir F, Friedman JM : The impact of array genomic hybridization on mental retardation research: a review of current technologies and their clinical utility. Clin Genet 2007; 72: 271–287.
Asadollahi R, Oneda B, Sheth F et al: Dosage changes of MED13L further delineate its role in congenital heart defects and intellectual disability. Eur J Hum Genet 2013; 21: 1100–1104.
van Haelst MM, Monroe GR, Duran K et al: Further confirmation of the MED13L haploinsufficiency syndrome. Eur J Hum Genet 2014; 23: 135–138.
Muncke N, Jung C, Rüdiger H et al: Missense variants and gene interruption in PROSIT240, a novel TRAP240-like gene, in patients with congenital heart defect (transposition of the great arteries). Circulation 2003; 108: 2843–2850.
Najmabadi H, Hu H, Garshasbi M et al: Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 2011; 478: 57–63.
Brazil A, Stanford K, Smolarek T, Hopkin R : Delineating the phenotype of 1p36 deletion in adolescents and adults. Am J Med Genet A 2014; 164A: 2496–2503.
Wang K, Li M, Hakonarson H : ANNOVAR: functional annotation of genetic variants from next-generation sequencing data. Nucleic Acids Res 2010; 38: e164.
Perin MS : Mirror image motifs mediate the interaction of the COOH terminus of multiple synaptotagmins with the neurexins and calmodulin. Biochemistry 1996; 35: 13808–13816.
Kochubey O, Lou X, Schneggenburger R : Regulation of transmitter release by Ca(2+) and synaptotagmin: insights from a large CNS synapse. Trends Neurosci 2011; 34: 237–246.
Yin JW, Wang G : The Mediator complex: a master coordinator of transcription and cell lineage development. Development 2014; 141: 977–987.
Angus SP, Nevins JR : A role for Mediator complex subunit MED13L in Rb/E2F-induced growth arrest. Oncogene 2012; 31: 4709–4717.
Gajecka M, Mackay KL, Shaffer LG : Monosomy 1p36 deletion syndrome. Am J Med Genet C Semin Med Genet 2007; 145C: 346–356.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cafiero, C., Marangi, G., Orteschi, D. et al. Novel de novo heterozygous loss-of-function variants in MED13L and further delineation of the MED13L haploinsufficiency syndrome. Eur J Hum Genet 23, 1499–1504 (2015). https://doi.org/10.1038/ejhg.2015.19
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2015.19
This article is cited by
-
Lethal variant in the C2A domain may cause severe SYT1-associated neurodevelopmental disorder in the newborns
neurogenetics (2023)
-
Report of a de novo c.2605C > T (p.Pro869Ser) change in the MED13L gene and review of the literature for MED13L-related intellectual disability
Italian Journal of Pediatrics (2020)
-
MED13L-related intellectual disability: involvement of missense variants and delineation of the phenotype
neurogenetics (2018)
-
Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases
Nature Genetics (2017)
-
De novo mutations in genes of mediator complex causing syndromic intellectual disability: mediatorpathy or transcriptomopathy?
Pediatric Research (2016)