Abstract
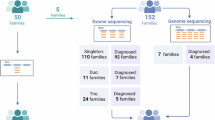
Heterozygous COL2A1 variants cause a wide spectrum of skeletal dysplasia termed type II collagenopathies. We assessed the impact of this gene in our French series. A decision tree was applied to select 136 probands (71 Stickler cases, 21 Spondyloepiphyseal dysplasia congenita cases, 11 Kniest dysplasia cases, and 34 other dysplasia cases) before molecular diagnosis by Sanger sequencing. We identified 66 different variants among the 71 positive patients. Among those patients, 18 belonged to multiplex families and 53 were sporadic. Most variants (38/44, 86%) were located in the triple helical domain of the collagen chain and glycine substitutions were mainly observed in severe phenotypes, whereas arginine to cysteine changes were more often encountered in moderate phenotypes. This series of skeletal dysplasia is one of the largest reported so far, adding 44 novel variants (15%) to published data. We have confirmed that about half of our Stickler patients (46%) carried a COL2A1 variant, and that the molecular spectrum was different across the phenotypes. To further address the question of genotype–phenotype correlation, we plan to screen our patients for other candidate genes using a targeted next-generation sequencing approach.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hoornaert KP, Vereecke I, Dewinter C et al: Stickler syndrome caused by COL2A1 mutations: genotype-phenotype correlation in a series of 100 patients. Eur J Hum Genet EJHG 2010; 18: 872–880.
Richards AJ, Laidlaw M, Whittaker J et al: High efficiency of mutation detection in type 1 stickler syndrome using a two-stage approach: vitreoretinal assessment coupled with exon sequencing for screening COL2A1. Hum Mutat 2006; 27: 696–704.
Pallares-Ruiz N, Philibert L, Dumont B et al: Combined mutation and rearrangement screening by quantitative PCR high-resolution melting: is it relevant for hereditary recurrent Fever genes? PloS One 2010; 5: e14096.
Mortier GR, Weis M, Nuytinck L et al: Report of five novel and one recurrent COL2A1 mutations with analysis of genotype-phenotype correlation in patients with a lethal type II collagen disorder. J Med Genet 2000; 37: 263–271.
Tysoe C, Saunders J, White L et al: A glycine to aspartic acid substitution of COL2A1 in a family with the Strudwick variant of spondyloepimetaphyseal dysplasia. QJM 2003; 96: 663–671.
Nishimura G, Haga N, Kitoh H et al: The phenotypic spectrum of COL2A1 mutations. Hum Mutat 2005; 26: 36–43.
Mark PR, Torres-Martinez W, Lachman RS, Weaver DD : Association of a p.Pro786Leu variant in COL2A1 with mild spondyloepiphyseal dysplasia congenita in a three-generation family. Am J Med Genet A 2011; 155A: 174–179.
Berg RA, Prockop DJ : The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun 1973; 52: 115–120.
Hoornaert KP, Dewinter C, Vereecke I et al: The phenotypic spectrum in patients with arginine to cysteine mutations in the COL2A1 gene. J Med Genet 2006; 43: 406–413.
Ala-Kokko L, Baldwin CT, Moskowitz RW, Prockop DJ : Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci USA 1990; 87: 6565–6568.
Eyre DR, Weis MA, Moskowitz RW : Cartilage expression of a type II collagen mutation in an inherited form of osteoarthritis associated with a mild chondrodysplasia. J Clin Invest 1991; 87: 357–361.
Ballo R, Beighton PH, Ramesar RS : Stickler-like syndrome due to a dominant negative mutation in the COL2A1 gene. Am J Med Genet 1998; 80: 6–11.
Richards AJ, McNinch A, Martin H et al: Stickler syndrome and the vitreous phenotype: mutations in COL2A1 and COL11A1. Hum Mutat 2010; 31: E1461–E1471.
Nagendran S, Richards AJ, McNinch A, Sandford RN, Snead MP : Somatic mosaicism and the phenotypic expression of COL2A1 mutations. Am J Med Genet A 2012; 158A: 1204–1207.
Chan D, Taylor TK, Cole WG : Characterization of an arginine 789 to cysteine substitution in alpha 1 (II) collagen chains of a patient with spondyloepiphyseal dysplasia. J Biol Chem 1993; 268: 15238–15245.
Donahue LR, Chang B, Mohan S et al: A missense mutation in the mouse Col2a1 gene causes spondyloepiphyseal dysplasia congenita, hearing loss, and retinoschisis. J Bone Miner Res Off J Am Soc Bone Miner Res 2003; 18: 1612–1621.
Zhang Z, He J-W, Fu W-Z, Zhang C-Q, Zhang Z-L : Identification of three novel mutations in the COL2A1 gene in four unrelated Chinese families with spondyloepiphyseal dysplasia congenita. Biochem Biophys Res Commun 2011; 413: 504–508.
Steplewski A, Ito H, Rucker E et al: Position of single amino acid substitutions in the collagen triple helix determines their effect on structure of collagen fibrils. J Struct Biol 2004; 148: 326–337.
Van Der Hout AH, Verlind E, Beemer FA, Buys CHCM, Hofstra RMW, Scheffer H : Occurrence of deletion of a COL2A1 allele as the mutation in Stickler syndrome shows that a collagen type II dosage effect underlies this syndrome. Hum Mutat 2002; 20: 236.
Miyake N, Tonoki H, Gallego M et al: Phenotype-genotype correlation in two patients with 12q proximal deletion. J Hum Genet 2004; 49: 282–284.
Yamanishi T, Nishio J, Miya S et al: 12q interstitial deletion with bilateral cleft lip and palate: case report and literature review. Cleft Palate Craniofac J 2008; 45: 325–328.
Gimelli S, Makrythanasis P, Stouder C, Antonarakis SE, Bottani A, Béna F : A de novo 12q13.11 microdeletion in a patient with severe mental retardation, cleft palate, and high myopia. Eur J Med Genet 2011; 54: 94–96.
Liberfarb RM, Levy HP, Rose PS et al: The Stickler syndrome: genotype/phenotype correlation in 10 families with Stickler syndrome resulting from seven mutations in the type II collagen gene locus COL2A1. Genet Med 2003; 5: 21–27.
Miyamoto Y, Nakashima E, Hiraoka H, Ohashi H, Ikegawa S : A type II collagen mutation also results in oto-spondylo-megaepiphyseal dysplasia. Hum Genet 2005; 118: 175–178.
Terhal PA, Nievelstein RJ, Verver EJ et al: A study of the clinical and radiological features in a cohort of 93 patients with a COL2A1 mutation causing spondyloepiphyseal dysplasia congenital or a related phenotype. Am J Med Genet A 2015; 167A: 461–475.
Kannu P, Irving M, Aftimos S, Savarirayan R : Two novel COL2A1 mutations associated with a Legg-Calvé-Perthes disease-like presentation. Clin Orthop Relat Res 2011; 469: 1785–1790.
Meredith SP, Richards AJ, Bearcroft P et al: Significant ocular findings are a feature of heritable bone dysplasias resulting from defects in type II collagen. Br J Ophthalmol 2007; 91: 1148–1151.
Richards AJ, Martin S, Yates JR et al: COL2A1 exon 2 mutations: relevance to the Stickler and Wagner syndromes. Br J Ophthalmol 2000; 84: 364–371.
Ahmad NN, Ala-Kokko L, Knowlton RG et al: Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy). Proc Natl Acad Sci USA 1991; 88: 6624–6627.
Hintze V, Steplewski A, Ito H, Jensen DA, Rodeck U, Fertala A : Cells expressing partially unfolded R789C/p.R989C type II procollagen mutant associated with spondyloepiphyseal dysplasia undergo apoptosis. Hum Mutat 2008; 29: 841–851.
Kuivaniemi H, Tromp G, Prockop DJ : Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat 1997; 9: 300–315.
Chan D, Cole WG : Low basal transcription of genes for tissue-specific collagens by fibroblasts and lymphoblastoid cells. Application to the characterization of a glycine 997 to serine substitution in alpha 1(II) collagen chains of a patient with spondyloepiphyseal dysplas. J Biol Chem 1991; 266: 12487–12494.
Zechi-Ceide RM, Jesus Oliveira NA, Guion-Almeida ML, Antunes LF, Richieri-Costa A, Passos-Bueno MR : Clinical evaluation and COL2A1 gene analysis in 21 Brazilian families with Stickler syndrome: identification of novel mutations, further genotype/phenotype correlation, and its implications for the diagnosis. Eur J Med Genet 2008; 51: 183–196.
Unger S, Korkko J, Krakow D et al: Double heterozygosity for pseudoachondroplasia and spondyloepiphyseal dysplasia congenita. Am J Med Genet 2001; 104: 140–146.
Acknowledgements
This work was supported by the CHRU de Montpellier. We thank the probands and their relatives for agreeing to participate in the molecular analysis and American Journal Experts for editing the manuscript for English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Barat-Houari, M., Dumont, B., Fabre, A. et al. The expanding spectrum of COL2A1 gene variants IN 136 patients with a skeletal dysplasia phenotype. Eur J Hum Genet 24, 992–1000 (2016). https://doi.org/10.1038/ejhg.2015.250
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2015.250
This article is cited by
-
Novel COL2A1 variants in Japanese patients with spondyloepiphyseal dysplasia congenita
Human Genome Variation (2022)
-
Expanding the clinical spectrum of COL2A1 related disorders by a mass like phenotype
Scientific Reports (2022)
-
A novel homozygous variant of COL2A1 in a Chinese male with type II collagenopathy: a case report
BMC Medical Genomics (2021)
-
Best practice guidelines in managing the craniofacial aspects of skeletal dysplasia
Orphanet Journal of Rare Diseases (2021)
-
Report on three additional patients and genotype–phenotype correlation in SLC25A22-related disorders group
European Journal of Human Genetics (2019)