Abstract
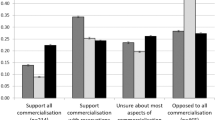
Many current paediatric studies concern relationships between genes and environment and discuss aetiology, treatment and prevention of Mendelian and multifactorial diseases. Many of these studies depend on collection and long-term storage of data and biological material from affected children in biobanks. Stored material is a source of personal information of the donor and his family and could be used in an undesirable context, potentially leading to discrimination and interfering with a child’s right to an open future. Here, we address the normative framework regarding biobanking with residual tissue of children, protecting the privacy interests of young biobank donors (0–12 years). We analyse relevant legal documents concerning storage and use of children’s material for research purposes. We explore the views of 17 Dutch experts involved in paediatric biobank research and focus on informed consent for donation of leftover tissue as well as disclosure of individual research findings resulting from biobank research. The results of this analysis show that experts have no clear consensus about the appropriate rules for storage of and research with children’s material in biobanks. Development of a framework that provides a fair balance between fundamental paediatric research and privacy protection is necessary.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Davis DS : Genetic dilemmas and the child's right to an open future. Rutgers Law J 1997; 28: 549–592.
Feinberg J : The child's right to an open future. In: Aiken W, LaFollette H (eds). Whose Child? Children's Rights, Parental Authority and State Power. Totowa, NJ: Rowman and Littlefield Adams, 1980, pp 124–153.
Wright J, Ploem C, Sliwka M, Gevers S : Regulating tissue research: do we need additional rules to protect research participants? Eur J Health Law 2010; 17: 455–469.
S. And Marper v. The United Kingdom: European Court of Human Rights, 2008.
Hens K, Nys H, Cassiman JJ, Dierickx K : Genetic research on stored tissue samples from minors: a systematic review of the ethical literature. Am J Med Genet Part A 2009; 149a: 2346–2358.
Gurwitz D, Fortier I, Lunshof JE, Knoppers BM : Research ethics. Children and population biobanks. Science (New York, NY) 2009; 325: 818–819.
Dutch Medical Treatment Contract Act, Wet op de geneeskundige behandelingsovereenkomst, 1995.
Hens K, Nys H, Cassiman JJ, Dierickx K : Biological sample collections from minors for genetic research: a systematic review of guidelines and position papers. Eur J Hum Genet 2009; 17: 979–990.
Hens K, Nys H, Cassiman JJ, Dierickx K : The storage and use of biological tissue samples from minors for research: a focus group study. Public Health Genom 2011; 14: 68–76.
Klima J, Fitzgerald-Butt SM, Kelleher KJ, et al: Understanding of informed consent by parents of children enrolled in a genetic biobank. Genet Med 2014; 16: 141–148.
Hens K, Dierickx K : The use of stored tissue samples from minors for genetic research: interviews with professionals. N Genet Soc 2010; 29: 329–342.
Polit DF, Beck CT : Nursing Research: Principles and Methods, 7th edn. Philadelphia, PA: Lippincott Williams & Wilkins, 2004.
Pope C, Ziebland S, Mays N : Qualitative research in health care; analysing qualitative data. BMJ 2000; 320: 114–116.
Council of Europe (CE) Convention for the Protection of Human Rights and Dignity of the Human Being With Regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine. Oviedo: Council of Europe, 1997, http://www.conventions.coe.int/Treaty/en/Treaties/Html/164.htm.
Council of Europe (CE) Explanatory Report to the Convention on Human Rights and Biomedicine. Strasbourg, France: Council of Europe, 1996, http://conventions.coe.int/Treaty/EN/Reports/Html/164.htm.
United Nations (UN) Convention on the Rights of the Child. New York, NY, USA: United Nations, 1989, http://www.ohchr.org/en/professionalinterest/pages/crc.aspx.
United Nations: Committee on the Rights of the child. General Comment No. 12, The Right of the Child to be Heard. Geneva, Switzerland: United Nations, 2009, http://www.refworld.org/cgi-bin/texis/vtx/rwmain?page=search&docid=4ae562c52&skip=0&query=general%20comment%2012.
Organisation for Economic Co-operation and Development (OECD) Guidelines on Human Biobanks and Genetic Research Databases (HBGRD). Paris, France: Organisation for Economic Co-operation and Development, 2009, http://www.oecd.org/science/biotech/44054609.pdf.
Council of Europe (CE) Recommendation R(2006)4 on Research on Biological Materials of Human Origin. Strasbourg, France: Council of Europe, 2006, https://wcd.coe.int/ViewDoc.jsp?id=977859.
Janssens ACJW : Raw personal data: access inaccuracy. Science (New York, NY) 2014; 343: 968.
Hens K, Nys H, Cassiman JJ, Dierickx K : Risks, benefits, solidarity: a framework for the participation of children in genetic biobank research. J Pediatr 2011; 158: 842–848.
Knoppers BM, Avard D, Cardinal G, Glass KC : Science and society: children and incompetent adults in genetic research: consent and safeguards. Nat Rev Genet 2002; 3: 221–225.
Hens K, Cassiman JJ, Nys H, Dierickx K : Children, biobanks and the scope of parental consent. Eur J Hum Genet 2011; 19: 735–739.
Giesbertz NA, Bredenoord AL, van Delden JJ : Consent procedures in pediatric biobanks. Eur J Hum Genet 2015; 23: 1129–1134.
Hansson MG, Dillner J, Bartram CR, Carlson JA, Helgesson G : Should donors be allowed to give broad consent to future biobank research? Lancet Oncol 2006; 7: 266–269.
Samuel J, Knoppers BM, Avard D : Paediatric biobanks: what makes them so unique? J Paediatr Child Health 2012; 48: E1–E3.
Brisson AR, Matsui D, Rieder MJ, Fraser DD : Translational research in pediatrics: tissue sampling and biobanking. Pediatrics 2012; 129: 153–162.
Harris ED, Ziniel SI, Amatruda JG, et al: The beliefs, motivations, and expectations of parents who have enrolled their children in a genetic biorepository. Genet Med 2012; 14: 330–337.
Dove ES, Avard D, Black L, Knoppers BM : Emerging issues in paediatric health research consent forms in Canada: working towards best practices. BMC Med Ethics 2013; 14: 5.
Hens K, Van El CE, Borry P, et al: Developing a policy for paediatric biobanks: principles for good practice. Eur J Hum Genet 2013; 21: 2–7.
Hens K, Levesque E, Dierickx K : Children and biobanks: a review of the ethical and legal discussion. Hum Genet 2011; 130: 403–413.
Helgesson G : Children, longitudinal studies, and informed consent. Med Health Care Philos 2005; 8: 307–313.
Holm S : Informed consent and the bio-banking of material from children. Genom Soc Pol 2005; 1: 16–26.
Knoppers BM, Avard D, Senecal K, Zawati MH : Return of whole-genome sequencing results in paediatric research: a statement of the P3G international paediatrics platform. Eur J Hum Genet 2014; 22: 3–5.
Avard D, Senecal K, Madadi P, Sinnett D : Pediatric research and the return of individual research results. J Law Med Ethics 2011; 39: 593–604.
Jarvik GP, Amendola LM, Berg JS, et al: Return of genomic results to research participants: the floor, the ceiling, and the choices in between. Am J Hum Genet 2014; 94: 818–826.
Dondorp WJ, de Wert GM : The 'thousand-dollar genome': an ethical exploration. Eur J Hum Genet 2013; 21 (Suppl 1): S6–26.
Van El CG, Cornel MC, Borry P, et al: Whole-genome sequencing in health care: recommendations of the European Society of Human Genetics. Eur J Hum Genet 2013; 21: 580–584.
Acknowledgements
This study was funded by the Netherlands Organisation for Health Research and Development (ZonMw). We thank the interviewees for offering us their insights and experiences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kranendonk, E., Ploem, M. & Hennekam, R. Regulating biobanking with children’s tissue: a legal analysis and the experts’ view. Eur J Hum Genet 24, 30–36 (2016). https://doi.org/10.1038/ejhg.2015.59
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2015.59
This article is cited by
-
Determining the state of guidance on pediatric biobanking for researchers, HRECS, and families: Regulatory mapping of international guidance
European Journal of Pediatrics (2024)
-
The general data protection regulation, the clinical trial regulation and some complex interplay in paediatric clinical trials
European Journal of Pediatrics (2021)
-
The Dutch legal approach regarding health care decisions involving minors in the NGS days
European Journal of Human Genetics (2017)
-
Paediatric biobanking: Dutch experts reflecting on appropriate legal standards for practice
European Journal of Pediatrics (2017)
-
Kinderbiobanken in Nederland
Tijdschrift voor gezondheidswetenschappen (2016)