Abstract
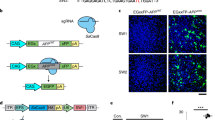
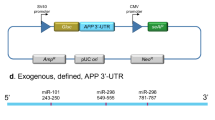
Aβ-related cerebral amyloid angiopathy (CAA) is a major cause of primary non-traumatic brain hemorrhage. In families with an early onset of the disease, CAA can be due to amyloid precursor protein (APP) pathogenic variants or duplications. APP duplications lead to a ~1.5-fold increased APP expression, resulting in Aβ overproduction and deposition in the walls of leptomeningeal vessels. We hypothesized that rare variants in the 3’untranslated region (UTR) of APP might lead to APP overexpression in patients with CAA and no APP pathogenic variant or duplication. We performed direct sequencing of the whole APP 3’UTR in 90 patients with CAA and explored the functional consequences of one previously unreported variant. We identified three sequence variants in four patients, of which a two-base pair deletion (c.*331_*332del) was previously unannotated and absent from 175 controls of same ethnicity. This latter variant was associated with increased APP expression in vivo and in vitro. Bioinformatics and functional assays showed that the APP c.*331_*332del variant affected APP messenger RNA (mRNA) structure and binding of two microRNAs (miR-582-3p and miR-892b), providing a mechanism for the observed effects on APP expression. These results identify APP 3’UTR sequence variants as genetic determinants of Aβ-CAA.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Greenberg SM, Vonsattel JP : Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke 1997; 28: 1418–1422.
Biffi A, Greenberg SM : Cerebral amyloid angiopathy: a systematic review. J Clin Neurol 2011; 7: 1–9.
Rannikmae K, Samarasekera N, Martinez-Gonzalez NA, Al-Shahi Salman R, Sudlow CL : Genetics of cerebral amyloid angiopathy: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2013; 84: 901–908.
McCarron MO, Nicoll JA : Apolipoprotein E genotype and cerebral amyloid angiopathy-related hemorrhage. Ann NY Acad Sci 2000; 903: 176–179.
Rovelet-Lecrux A, Hannequin D, Raux G et al: APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 2006; 38: 24–26.
Pottier C, Wallon D, Lecrux AR et al: Amyloid-beta protein precursor gene expression in alzheimer's disease and other conditions. J Alzheimers Dis 2012; 28: 561–566.
Donahue JE, Khurana JS, Adelman LS : Intracerebral hemorrhage in two patients with Down's syndrome and cerebral amyloid angiopathy. Acta Neuropathol 1998; 95: 213–216.
Bettens K, Brouwers N, Engelborghs S et al: APP and BACE1 miRNA genetic variability has no major role in risk for Alzheimer disease. Hum Mutat 2009; 30: 1207–1213.
Delay C, Calon F, Mathews P, Hebert SS : Alzheimer-specific variants in the 3'UTR of Amyloid precursor protein affect microRNA function. Mol Neurodegener 2011; 6: 70.
Filipowicz W, Bhattacharyya SN, Sonenberg N : Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 2008; 9: 102–114.
Hebert SS, De Strooper B : Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci 2009; 32: 199–206.
Hebert SS, Nelson PT : Studying microRNAs in the brain: technical lessons learned from the first ten years. Exp Neurol 2012; 235: 397–401.
Linn J, Halpin A, Demaerel P et al: Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010; 74: 1346–1350.
Guerchet M, Mbelesso P, Ndamba-Bandzouzi B et al: Epidemiology of dementia in Central Africa (EPIDEMCA): Protocol for a multicentre population-based study in rural and urban areas of the Central African Republic and the Republic of Congo. Springerplus 2014; 3: 338.
Bindewald E, Kluth T, Shapiro BA : CyloFold: secondary structure prediction including pseudoknots. Nucleic Acids Res 2010; 38: W368–W372.
Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E : The role of site accessibility in microRNA target recognition. Nat Genet 2007; 39: 1278–1284.
Hebert SS, Horre K, Nicolai L et al: MicroRNA regulation of Alzheimer's Amyloid precursor protein expression. Neurobiol Dis 2009; 33: 422–428.
Dorval V, Mandemakers W, Jolivette F et al: Gene and MicroRNA transcriptome analysis of Parkinson's related LRRK2 mouse models. PloS One 2014; 9: e85510.
Hebert SS, Wang WX, Zhu Q, Nelson PT : A study of small RNAs from cerebral neocortex of pathology-verified Alzheimer's disease, dementia with lewy bodies, hippocampal sclerosis, frontotemporal lobar dementia, and non-demented human controls. J Alzheimers Dis 2013; 35: 335–348.
Theuns J, Brouwers N, Engelborghs S et al: Promoter mutations that increase amyloid precursor-protein expression are associated with Alzheimer disease. Am J Hum Genet 2006; 78: 936–946.
Lahiri DK, Ge YW, Maloney B, Wavrant-De Vrieze F, Hardy J : Characterization of two APP gene promoter polymorphisms that appear to influence risk of late-onset Alzheimer's disease. Neurobiol Aging 2005; 26: 1329–1341.
Guyant-Marechal L, Rovelet-Lecrux A, Goumidi L et al: Variations in the APP gene promoter region and risk of Alzheimer disease. Neurology 2007; 68: 684–687.
Nowotny P, Simcock X, Bertelsen S et al: Association studies testing for risk for late-onset Alzheimer's disease with common variants in the beta-amyloid precursor protein (APP). Am J Med Genet B Neuropsychiatr Genet 2007; 144B: 469–474.
Delay C, Mandemakers W, Hebert SS : MicroRNAs in Alzheimer's disease. Neurobiol Dis 2012; 46: 285–290.
Long JM, Ray B, Lahiri DK : MicroRNA-153 physiologically inhibits expression of amyloid-beta precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J Biol Chem 2012; 287: 31298–31310.
Acknowledgements
We thank all physicians who sent patient information and blood samples. This work was supported by the CNR-MAJ with grants from the French Ministry of Health, the University Hospital of Rouen, the Alzheimer Society of Canada, the Canadian Institutes of Health Research, and the AP-HP Lariboisière Hospital of Paris.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nicolas, G., Wallon, D., Goupil, C. et al. Mutation in the 3’untranslated region of APP as a genetic determinant of cerebral amyloid angiopathy. Eur J Hum Genet 24, 92–98 (2016). https://doi.org/10.1038/ejhg.2015.61
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2015.61
This article is cited by
-
A Rare Variation in the 3’ Untranslated Region of the Presenilin 2 Gene Is Linked to Alzheimer’s Disease
Molecular Neurobiology (2021)
-
Alzheimer’s genetic risk factor FERMT2 (Kindlin-2) controls axonal growth and synaptic plasticity in an APP-dependent manner
Molecular Psychiatry (2021)
-
Cerebral Amyloid Angiopathy, Alzheimer’s Disease and MicroRNA: miRNA as Diagnostic Biomarkers and Potential Therapeutic Targets
NeuroMolecular Medicine (2019)
-
Patterns and severity of vascular amyloid in Alzheimer’s disease associated with duplications and missense mutations in APP gene, Down syndrome and sporadic Alzheimer’s disease
Acta Neuropathologica (2018)
-
miRNA-dependent target regulation: functional characterization of single-nucleotide polymorphisms identified in genome-wide association studies of Alzheimer’s disease
Alzheimer's Research & Therapy (2016)