Abstract
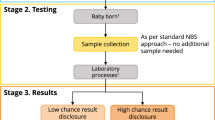
Consent processes for newborn bloodspot screening (NBS) are variable, with a lack of descriptive research that depicts how the offer of NBS is made to parents. We explored the experience, in practice, of consent for NBS. Semistructured interviews in two Canadian provinces were held with: (1) parents of children offered NBS (n=32); and (2) health-care professionals involved in the NBS process (n=19). Data on recollections of NBS, including consent processes, were utilized to identify emerging themes using the method of constant comparison. Three themes were relevant to NBS consent: (1) The ‘offer’ of NBS; (2) content and timing of information provision; and (3) the importance of parental experiences for consent decisions. Recollections of consent for NBS were similar between jurisdictions. Excepting midwives and their patients, NBS was viewed as a routine part of giving birth, with little evidence of an informed consent process. Although most parents were satisfied, all respondents suggested information about NBS be provided long before the birth. Accounts of parents who declined screening highlight the influence of parental experiences with the heel prick process in screening decisions. Findings further our understanding of consent in practice and highlight areas for improvement in parent–provider interactions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Padilla CD, Krotoski D, Therrell BL Jr : Newborn screening progress in developing countries - overcoming internal barriers. Semin Perinatol 2010; 34: 145–155.
Padilla CD, Therrell BL Jr : Consolidating newborn screening efforts in the Asia Pacific region: networking and shared education. J Community Genet 2012; 3: 35–45.
Pollitt RJ : International perspectives on newborn screening. J Inherit Metab Dis 2006; 29: 390–396.
Therrell BL, Padilla CD, Loeber JG et al: Current status of newborn screening worldwide: 2015. Semin Perinatol 2015; 39: 171–187.
Grosse SD, Boyle CA, Kenneson A, Khoury MJ, Wilfond BS : From public health emergency to public health service: the implications of evolving criteria for newborn screening panels. Pediatrics 2006; 117: 923–929.
Miller FA, Hayeems RZ, Carroll JC et al: Consent for newborn screening: the attitudes of health care providers. Public Health Genomics 2010; 13: 181–190.
Botkin JR, Lewis MH, Watson MS et al: Parental permission for pilot newborn screening research: guidelines from the NBSTRN. Pediatrics 2014; 133: e410–e417.
Pollitt RJ : Introducing new screens: why are we all doing different things? J Inherit Metab Dis 2007; 30: 423–429.
Clayton EW : Talking with parents before newborn screening. J Pediatr 2005; 147: S26–S29.
Bailey DB Jr, Beskow LM, Davis AM, Skinner D : Changing perspectives on the benefits of newborn screening. Ment Retard Dev Disabil Res Rev 2006; 12: 270–279.
Araia MH, Potter BK : Newborn screening education on the internet: a content analysis of North American newborn screening program websites. J Community Genet 2011; 2: 127–134.
Fox R : Informed choice in screening programmes: do leaflets help? A critical literature review. J Public Health (Oxf) 2006; 28: 309–317.
Hargreaves K, Stewart R, Oliver S : Newborn screening information supports public health more than informed choice. Health Educ J 2005; 64: 110–119.
Nicholls SG : Proceduralisation, choice and parental reflections on decisions to accept newborn bloodspot screening. J Med Ethics 2012; 38: 299–303.
Muchamore I, Morphett L, Barlow-Stewart K : Exploring existing and deliberated community perspectives of newborn screening: informing the development of state and national policy standards in newborn screening and the use of dried blood spots. Aust New Zealand Health Policy 2006; 3: 14.
Araia MH, Wilson BJ, Chakraborty P et al: Factors associated with knowledge of and satisfaction with newborn screening education: a survey of mothers. Genet Med 2012; 14: 963–970.
Potter BK, Etchegary H, Nicholls SG, Wilson BJ, Craigie SM, Araia MH : Education and parental involvement in decision-making about newborn screening: understanding goals to clarify content. J Genet Couns 2015; 24: 400–408.
Bombard Y, Miller FA, Hayeems RZ et al: Public views on participating in newborn screening using genome sequencing. Eur J Hum Genet 2014; 22: 1248–1254.
Ross LF : Mandatory versus voluntary consent for newborn screening? Kennedy Inst Ethics J 2010; 20: 299–328.
Therrell BL, Johnson A, Williams D : Status of newborn screening programs in the United States. Pediatrics 2006; 117: S212–S252.
Ross LF, Saal HM, David KL, Anderson RR, American Academy of Pediatrics; American College of Medical Genetics and Genomics: Technical report: ethical and policy issues in genetic testing and screening of children. Genet Med 2013; 15: 234–245.
UK Newborn Screening Programme Centre Guidelines for Newborn Blood Spot Sampling. London, UK: UK National Screening Committee, 2012.
Nicholls SG, Wilson BJ, Etchegary H, Potter B, Carroll J : Benefits and burdens of newborn screening: public understanding and decision-making. Personal Med 2014; 11: 593–607.
Grady C : Enduring and emerging challenges of informed consent. N Engl J Med 2015; 372: 855–862.
Campbell E, Ross LF : Parental attitudes regarding newborn screening of PKU and DMD. Am J Med Genet 2003; 120: 209–214.
Davey A, French D, Dawkins H, O'Leary P : New mothers' awareness of newborn screening, and their attitudes to the retention and use of screening samples for research purposes. Genomics Soc Policy 2006; 1: 41–51.
Detmar S, Hosli E, Dijkstra N, Nijsingh N, Rijnders M, Verweij M : Information and informed consent for neonatal screening: opinions and preferences of parents. Birth 2007; 34: 238–244.
Hasegawa LE, Fergus KA, Ojeda N, Au SM : Parental attitudes toward ethical and social issues surrounding the expansion of newborn screening using new technologies. Public Health Genomics 2010; 14: 298–306.
Hayeems RZ, Miller FA, Bombard Y et al: Expectations and values about expanded newborn screening: a public engagement study. Health Expect 2015; 18: 419–429.
Kerruish NJ, Webster D, Dickson N : Information and consent for newborn screening: practices and attitudes of service providers. J Med Ethics 2008; 34: 648–652.
Hargreaves K, Stewart R, Oliver S : Informed choice and public health screening for children: the case of blood spot screening. Health Expect 2005; 8: 161–171.
Nicholls SG, Tessier L, Etchegary H et al: Stakeholder attitudes towards the role and application of informed consent for newborn bloodspot screening: a study protocol. BMJ Open 2014; 4: e006782.
Sandelowski M : Whatever happened to qualitative description? Res Nurs Health 2000; 23: 334–340.
Pope C, Ziebland S, Mays N : Analysing qualitative data. BMJ 2000; 320: 114–116.
Lipstein EA, Nabi E, Perrin JM, Luff D, Browning MF, Kuhlthau KA : Parents' decision-making in newborn screening: opinions, choices, and information needs. Pediatrics 2010; 126: 696–704.
Moody L, Choudhry K : Parental views on informed consent for expanded newborn screening. Health Expect 2011; 16: 239–250.
Green JM, Hewison J, Bekker HL, Bryant LD, Cuckle HS : Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review. Health Tech Assess 2004; 8: 1–109.
Gray L, Watt L, Blass EM : Skin-to-skin contact is analgesic in healthy newborns. Pediatrics 2000; 105: e14.
Gray L, Miller LW, Philipp BI, Blass EM : Breastfeeding is analgesic in healthy newborns. Pediatrics 2002; 109: 590–593.
Newborn Screening Ontario Newborn Screening Manual. A Guide for Newborn Care Providers. Ottawa, Canada: Newborn Screening Ontario, 2015.
Acknowledgements
This work is funded by the Canadian Institutes of Health Research through the (then) Open Operating grant competition, reference number EOG-131589. We thank interview respondents for their time and input.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Etchegary, H., Nicholls, S., Tessier, L. et al. Consent for newborn screening: parents’ and health-care professionals’ experiences of consent in practice. Eur J Hum Genet 24, 1530–1534 (2016). https://doi.org/10.1038/ejhg.2016.55
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2016.55
This article is cited by
-
Application of machine learning algorithms for accurate determination of bilirubin level on in vitro engineered tissue phantom images
Scientific Reports (2024)
-
Pain points in parents’ interactions with newborn screening systems: a qualitative study
BMC Pediatrics (2022)
-
Regulatory landscape of providing information on newborn screening to parents across Europe
European Journal of Human Genetics (2021)