Abstract
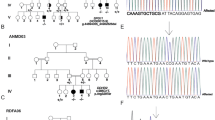
Twelve individuals of consanguineous Bedouin kindred presented with autosomal recessive progressive spastic paraplegia evident as of age 0–24 months, with spasticity of lower limbs, hyperreflexia, toe walking and equinus deformity. Kyphoscolisois was evident in older patients. Most had atrophy of the lateral aspects of the tongue and few had intellectual disability. Nerve conduction velocity, electromyography and head and spinal cord magnetic resonance imaging were normal in tested subjects. Muscle biopsy showed occasional central nuclei and fiber size variability with small angular fibers. Genome-wide linkage analysis identified a 6.7Mbp disease-associated locus on chromosome 3q21.3–3q22.2 (LOD score 9.02; D3S1290). Whole-exome sequencing identified a single homozygous variant within this locus, c.51_52ins(28); p.(V18fs56*) in KY, segregating in the family as expected and not found in 190 Bedouin controls. High KY transcript levels were demonstrated in muscular organs with lower expression in the CNS. The phenotype is reminiscent of kyphoscoliosis seen in Ky null mice. Two recent studies done independently and parallel to ours describe somewhat similar phenotypes in one and two patients with KY mutations. KY encodes a tranglutaminase-like peptidase, which interacts with muscle cytoskeletal proteins and is part of a Z-band protein complex, suggesting the disease mechanism may resemble myofibrillar myopathy. However, the mixed myopathic–neurologic features caused by human and mouse Ky mutations are difficult to explain by loss of KY sarcomere stabilizing function alone. KY transcription in CNS tissues may imply that it also has a role in neuromotor function, in line with the irregularity of neuromuscular junction in Ky null mutant mice.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fink JK : Hereditary Spastic Paraplegia Overview. Seattle: University of Washington, 2014, http://www.ncbi.nlm.nih.gov/pubmed/20301682 (accessed 25 November 2016).
de Souza PV, de Rezende Pinto WB, de Rezende Batistella GN, Bortholin T, Oliveira AS : Hereditary spastic paraplegia: clinical and genetic hallmarks. Cerebellum 2016; 16: 525–551.
Finsterer J, Löscher W, Quasthoff S, Wanschitz J, Michaela A-G, Stevanin G : Hereditary spastic paraplegias with autosomal dominant, recessive, X-linked, or maternal trait of inheritance. J Neurol Sci 2012; 318: 1–18.
Seelow D, Schuelke M, Hildebrandt F, Nurnberg P : HomozygosityMapper—an interactive approach to homozygosity mapping. Nucleic Acids Res 2009; 37: W593–W599.
Perez Y, Gradstein L, Flusser H et al: Isolated foveal hypoplasia with secondary nystagmus and low vision is associated with a homozygous SLC38A8 mutation. Eur J Hum Genet 2014; 22: 703–706.
Silberstein M, Weissbrod O, Otten L et al: A system for exact and approximate genetic linkage analysis of SNP data in large pedigrees. Bioinformatics 2013; 29: 197–205.
Dickinson AG, Meikle VM : Genetic kyphoscoliosis in mice. Lancet (London, England) 1973; 1: 1186.
Blanco G, Coulton GR, Biggin A et al: The kyphoscoliosis (ky) mouse is deficient in hypertrophic responses and is caused by a mutation in a novel muscle-specific protein. Hum Mol Genet 2001; 10: 9–16.
Bridges LR, Coulton GR, Howard G, Moss J, Mason RM : The neuromuscular basis of hereditary kyphoscoliosis in the mouse. Muscle Nerve 1992; 15: 172–179.
Blanco G, Pritchard C, Underhill P et al: Molecular phenotyping of the mouse ky mutant reveals {UCP1} upregulation at the neuromuscular junctions of dystrophic soleus muscle. Neuromuscul Disord 2004; 14: 217–228.
Folker ES, Baylies MK : Nuclear positioning in muscle development and disease. Front Physiol 2013; 4: 363.
Doppler K, Mittelbronn M, Bornemann A : Myogenesis in human denervated muscle biopsies. Muscle Nerve 2008; 37: 79–83.
Altschul SF, Madden TL, Schäffer AA et al: Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997; 25: 3389–3402.
Carola H-O, Darin N, Engman M et al: A new early-onset neuromuscular disorder associated with kyphoscoliosis peptidase KY deficiency. Eur J Hum Genet 2016; 24: 1771–1777.
Straussberg R, Schottmann G, Sadeh M et al: Kyphoscoliosis peptidase {(KY)} mutation causes a novel congenital myopathy with core targetoid defects. Acta Neuropathol 2016; 132: 475–478.
Pereira FJC, Teixeira A, Kong J et al: Resistance of mRNAs with AUG-proximal nonsense mutations to nonsense-mediated decay reflects variables of mRNA structure and translational activity. Nucleic Acids Res 2015; 43: 6528–6544.
Beatham J, Romero R, Townsend S, Hacker T, van der Ven P, Blanco G : Filamin C interacts with the muscular dystrophy {KY} protein and is abnormally distributed in mouse {KY} deficient muscle fibres. Hum Mol Genet 2004; 13: 2863–2874.
Baker J, Riley G, Romero RM et al: Identification of a Z-band associated protein complex involving {KY,} {FLNC} and {IGFN1}. Exp Cell Res 2010; 316: 1856–1870.
Trotta N, Orso G, Rossetto MG, Daga A, Broadie K : The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr Biol 2004; 14: 1135–1147.
Gan-Or Z, Bouslam N, Birouk N et al: Mutations in CAPN1 cause autosomal-recessive hereditary spastic paraplegia. Am J Hum Genet 2016; 98: 1038–1046.
Acknowledgements
Funding for this research was provided by the Legacy Heritage Biomedical Program of the Israel Science Foundation (grant 1798/16 awarded to OSB and grant 1520/09 awarded to OSB and RB); and by a grant to OSB and scholarship to YY, both by Teva Pharmaceutical Industries Ltd as part of the Israeli National Network of Excellence in Neuroscience (NNE). We thank the patients and their families for their efforts and collaboration in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yogev, Y., Perez, Y., Noyman, I. et al. Progressive hereditary spastic paraplegia caused by a homozygous KY mutation. Eur J Hum Genet 25, 966–972 (2017). https://doi.org/10.1038/ejhg.2017.85
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2017.85
This article is cited by
-
Long-term course of a case with a novel homozygous kyphoscoliosis peptidase variant
Journal of Human Genetics (2024)
-
Tissue-aware interpretation of genetic variants advances the etiology of rare diseases
Molecular Systems Biology (2024)
-
SCAPER localizes to primary cilia and its mutation affects cilia length, causing Bardet-Biedl syndrome
European Journal of Human Genetics (2019)