Abstract
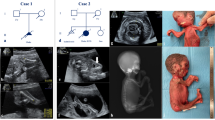
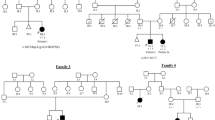
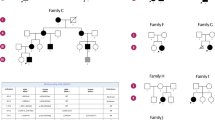
Heterozygous variants in BICD cargo adapter 2 (BICD2) cause autosomal dominant spinal muscular atrophy, lower extremity-predominant 2 (SMALED2). The disease is usually characterized by a benign or slowly progressive, congenital or early onset muscle weakness and atrophy that mainly affects the lower extremities, although some affected individuals show involvement of the arms and the shoulder girdle. Here we report unusual extremes of BICD2-related diseases: A severe form of congenital muscular atrophy with arthrogryposis multiplex, respiratory insufficiency and lethality within four months. This was caused by three BICD2 variants, (c.581A>G, p.(Gln194Arg)), (c.1626C>G, p.(Cys542Trp)) and (c.2080C>T, p.(Arg694Cys)), two of which were proven to be de novo. Affected individuals showed reduced fetal movement, weak muscle tone and sparse or no spontaneous activity after birth. Despite assisted ventilation, the condition led to early death. At the other extreme, we identified an asymptomatic woman with a known BICD2 variant (c.2108C>T, p.(Thr703Met)). Radiological examination showed fatty degeneration of selected thigh and calf muscles without clinical consequences. Instead, her son carrying the same variant is affected by a mild childhood onset disease with myopathic and neurogenic features. Mechanisms leading to variable expressivity and onset of BICD2-related disease may include alterations in molecular interactions of BICD2 and suggest the presence of genetic modifiers that may act in a protective fashion to ameliorate or abrogate disease. Our data define an additional severe disease type caused by BICD2 and emphasize a possibly variable etiology of BICD2-opathies with regard to primary muscle and neuronal involvement.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Neveling K, Martinez-Carrera LA, Holker I et al: Mutations in BICD2, which encodes a golgin and important motor adaptor, cause congenital autosomal-dominant spinal muscular atrophy. Am J Hum Genet 2013; 96: 946–954.
Peeters K, Litvinenko I, Asselbergh B et al: Molecular defects in the motor adaptor BICD2 cause proximal spinal muscular atrophy with autosomal-dominant inheritance. Am J Hum Genet 2013; 92: 955–964.
Oates EC, Rossor AM, Hafezparast M et al: Mutations in BICD2 cause dominant congenital spinal muscular atrophy and hereditary spastic paraplegia. Am J Hum Genet 2013; 92: 965–973.
Rossor AM, Oates EC, Salter HK et al: Phenotypic and molecular insights into spinal muscular atrophy due to mutations in BICD2. Brain 2015; 138: 293–310.
Synofzik M, Martinez-Carrera LA, Lindig T, Schols L, Wirth B : Dominant spinal muscular atrophy due to BICD2: a novel mutation refines the phenotype. J Neurol Neurosurg Psychiatry 2014; 85: 590–592.
Rudnik-Schoneborn S, Deden F, Eggermann K et al: Autosomal dominant spinal muscular atrophy with lower extremity predominance: a recognizable phenotype of BICD2 mutations. Muscle Nerve 2016; 54: 496–500.
Hoogenraad CC, Akhmanova A, Howell SA et al: Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes. EMBO J 2001; 20: 4041–4054.
Matanis T, Akhmanova A, Wulf P et al: Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol 2002; 4: 986–992.
Bredrup C, Johansson S, Bindoff LA et al: High myopia-excavated optic disc anomaly associated with a frameshift mutation in the MYC-binding protein 2 gene (MYCBP2). Am J Ophthalmol 2015; 159: 973–979, e972.
Unger A, Dekomien G, Guttsches A et al: Expanding the phenotype of BICD2 mutations toward skeletal muscle involvement. Neurology 2016; 87: 2235–2243.
Ravenscroft G, Di Donato N, Hahn G et al: Recurrent de novo BICD2 mutation associated with arthrogryposis multiplex congenita and bilateral perisylvian polymicrogyria. Neuromuscul Disord 2016; 26: 744–748.
Adams C, Suchowersky O, Lowry RB : Congenital autosomal dominant distal spinal muscular atrophy. Neuromuscul Disord 1998; 8: 405–408.
Bamshad M, Van Heest AE : Pleasure D: Arthrogryposis: a review and update. J Bone Joint Surg Am 2009; 91: 40–46.
Abicht A, Muller JS, Lochmuller H Congenital Myasthenic Syndromes. In: Pagon RA, Adam MP, Ardinger HH et al(eds): GeneReviews(R) 1993.
Burglen L, Amiel J, Viollet L et al: Survival motor neuron gene deletion in the arthrogryposis multiplex congenita-spinal muscular atrophy association. J Clin Invest 1996; 98: 1130–1132.
Ramser J, Ahearn ME, Lenski C et al: Rare missense and synonymous variants in UBE1 are associated with X-linked infantile spinal muscular atrophy. Am J Hum Genet 2008; 82: 188–193.
Hall JG : Arthrogryposis multiplex congenita: etiology, genetics, classification, diagnostic approach, and general aspects. J Pediatr Orthop B 1997; 6: 159–166.
Fiorillo C, Moro F, Brisca G et al: Beyond spinal muscular atrophy with lower extremity dominance: cerebellar hypoplasia associated with a novel mutation in BICD2. Eur J Neurol 2016; 23: e19–e21.
Martinez-Carrera LA, Wirth B : Dominant spinal muscular atrophy is caused by mutations in BICD2, an important golgin protein. Front Neurosci 2015; 9: 401.
Splinter D, Razafsky DS, Schlager MA et al: BICD2, dynactin, and LIS1 cooperate in regulating dynein recruitment to cellular structures. Mol Biol Cell 2012; 23: 4226–4241.
Terenzio M, Schiavo G : The more, the better: the BICD family gets bigger. EMBO J 2010; 29: 1625–1626.
Poirier K, Lebrun N, Broix L et al: Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat Genet 2013; 45: 639–647.
Schiavo G, Greensmith L, Hafezparast M, Fisher EM : Cytoplasmic dynein heavy chain: the servant of many masters. Trends Neurosci 2013; 36: 641–651.
Hoang HT, Schlager MA, Carter AP, Bullock SL : DYNC1H1 mutations associated with neurological diseases compromise processivity of dynein-dynactin-cargo adaptor complexes. Proc Natl Acad Sci USA 2017; 9: e1597–e1606.
Harms MB, Ori-McKenney KM, Scoto M et al: Mutations in the tail domain of DYNC1H1 cause dominant spinal muscular atrophy. Neurology 2012; 78: 1714–1720.
Kousi M, Katsanis N : Genetic modifiers and oligogenic inheritance. Cold Spring Harb Perspect Med 2015; 5: a017145.
Hosseinibarkooie S, Peters M, Torres-Benito L et al: The power of human protective modifiers: PLS3 and CORO1C unravel impaired endocytosis in spinal muscular atrophy and rescue SMA phenotype. Am J Hum Genet 2016; 99: 647–665.
Riessland M, Kaczmarek A, Schneider S et al: Neurocalcin delta suppression protects against spinal muscular atrophy in humans and across species by restoring impaired endocytosis. Am J Hum Genet 2017; 100: 297–315.
Acknowledgements
We are grateful to the families, who supported this study. The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 2012-305121 “Integrated European –omics research project for diagnosis and therapy in rare neuromuscular and neurodegenerative diseases (NEUROMICS)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Storbeck, M., Horsberg Eriksen, B., Unger, A. et al. Phenotypic extremes of BICD2-opathies: from lethal, congenital muscular atrophy with arthrogryposis to asymptomatic with subclinical features. Eur J Hum Genet 25, 1040–1048 (2017). https://doi.org/10.1038/ejhg.2017.98
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2017.98
This article is cited by
-
Missense BICD2 variants in fetuses with congenital arthrogryposis and pterygia
Human Genome Variation (2024)
-
A homozygous loss-of-function variant in BICD2 is associated with lissencephaly and cerebellar hypoplasia
Journal of Human Genetics (2022)
-
Loss of BICD2 in muscle drives motor neuron loss in a developmental form of spinal muscular atrophy
Acta Neuropathologica Communications (2020)
-
Impairment in dynein-mediated nuclear translocation by BICD2 C-terminal truncation leads to neuronal migration defect and human brain malformation
Acta Neuropathologica Communications (2020)
-
Dynein activating adaptor BICD2 controls radial migration of upper-layer cortical neurons in vivo
Acta Neuropathologica Communications (2019)