Abstract
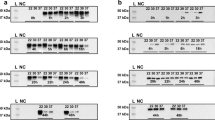
Five monoclonal antibodies (mAbs) that recognize human glutamate dehydrogenase (GDH) have been selected and designated as monoclonal antibodies hGDH60-6, hGDH60-8, hGDH63-10, hGDH63-11, and hGDH91-14. A total of five mAbs recognizing different epitopes of the enzyme were obtained, two of which inhibited human GDH activity. When total proteins of human homogenate separated by SDS- PAGE, were probed with mAbs, a single reactive protein band of 55 kDa, which co-migrated with purified recombinant human GDH was detected. When the purified GDH was incubated with each of the mAbs, its enzyme activity was inhibited by up to 58%. Epitope mapping analysis identified, two subgroups of mAbs recognizing different peptide fragments. Using the individual anti-GDH antibodies as probes, the cross reactivities of brain GDH obtained from human and other animal brain tissues were investigated. For the human and animal tissues tested, immunoreactive bands on Western blots appeared to have the same molecular mass of 55 kDa when hGHD60-6, hGHD60-8, or hGHD91-14 mAbs were used as probes. However, the anti-human GDH mAbs immunoreactive to bands on Western blots reacted differently on the immunoblots of the other animal brains tested, i.e., the two monoclonal antibodies hGDH63-10 and hGDH63-11 only produced positive results for human. These results suggest that human brain GDH is immunologically distinct from those of other mammalian brains. Thorough characterization of these anti-human GDH mAbs could provide potentially valuable tool as immunodiagnostic reagents for the detection, identification and characterization of the various neurological diseases related to the GDH enzyme.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Jang, S., Kim, A., Bahn, J. et al. Human glutamate dehydrogenase is immunologically distinct from other mammalian orthologues. Exp Mol Med 35, 249–256 (2003). https://doi.org/10.1038/emm.2003.33
Published:
Issue date:
DOI: https://doi.org/10.1038/emm.2003.33