Abstract
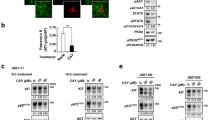
Agents that elevate cellular cAMP are known to inhibit the activation of phospholipase D (PLD). We investigated whether PLD can be phosphorylated by cAMP-dependent protein kinase (PKA) and PKA-mediated phosphorylation affects the interaction between PLD and RhoA, a membrane regulator of PLD. PLD1, but not PLD2 was found to be phosphorylated in vivo by the treatment of dibutyryl cAMP (dbcAMP) and in vitro by PKA. PKA inhibitor (KT5720) abolished the dbcAMP-induced phosphorylation of PLD1, but dibutyryl cGMP (dbcGMP) failed to phosphorylate PLD1. The association between PLD1 and Val14RhoA in an immunoprecipitation assay was abolished by both dbcAMP and dbcGMP. Moreover, RhoA but not PLD1 was dissociated from the membrane to the cytosolic fraction in dbcAMP-treated cells. These results suggest that both PLD1 and RhoA are phosphorylated by PKA and the interaction between PLD1 and RhoA is inhibited by the phosphorylation of RhoA rather than by the phosphorylation of PLD1.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Jang, MJ., Lee, MJ., Park, HY. et al. Phosphorylation of phospholipase D1 and the modulation of its interaction with RhoA by cAMP-dependent protein kinase. Exp Mol Med 36, 172–178 (2004). https://doi.org/10.1038/emm.2004.24
Published:
Issue date:
DOI: https://doi.org/10.1038/emm.2004.24
Keywords
This article is cited by
-
A comprehensive pathway map of epidermal growth factor receptor signaling
Molecular Systems Biology (2005)