Abstract
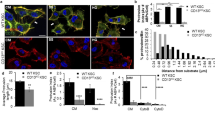
p21-activated kinase (PAK)-interacting exchange factor (PIX) is known to be involved in regulation of Cdc42/Rac GTPases and PAK activity. PIX binds to the proline-rich region of PAK, and regulates biological events through activation of Cdc42/Rac GTPase. To further investigate the role of PIX we produced monoclonal antibodies (Mab) against βPIK. Three clones; N-C6 against N-terminal half and C-A3 and C-B7 against C- terminal half of βPIK were generated and characterized. N-C6 Mab detected βPIK as a major band in most cell lines. C-A3 Mab recognizes GIT-binding domain (GBD), but it does not interfere with GIT binding to βPIK. Using C-A3 Mab possible βPIK interaction with actin in PC12 cells was examined. βPIK Mab (C-A3) specifically precipitated actin of the PC12 cell lysates whereas actin Mab failed to immunoprecpitate βPIK. Co-sedimentation of PC12 cell lysates with the polymerized F-actin resulted in the recovery of most of βPIK in the cell lysates. These results suggest that βPIK may not interact with soluble actin but with polymerized F-actin and revealed that βPIK constitutes a functional complex with actin. These data indicate real usefulness of the βPIK Mab in the study of βPIK role(s) in regulation of actin cyoskeleton.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lee, CS., Kim, KY., Im, JB. et al. β PAK-interacting exchange factor may regulate actin cytoskeleton through interaction with actin. Exp Mol Med 36, 582–587 (2004). https://doi.org/10.1038/emm.2004.75
Published:
Issue date:
DOI: https://doi.org/10.1038/emm.2004.75